
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
What would the mechanism look like below?
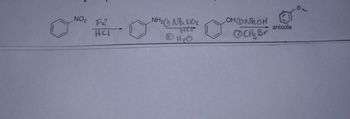
Transcribed Image Text:NO₂ 2
HCT
NH₂
H₂ NaNO₂
HICH
H₂O
LOHODNAOH
CH₂ B
anisole
¡
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain how you would select the optimal average linear velocity for these two gases. Which carrier gas would have better resolution at twice optimal linear velocity and why?arrow_forwardWhen selecting a ball from your model kit to serve as the phosphorous atom from above, how many holes should the ball have? Why?arrow_forwardAmmonia gas is produced from nitrogen gas and hydrogen gas. A. Write a balanced chemical equation for this reaction. B. At STP, what mass (in grams) of nitrogen is needed for the complete reaction of 15.1 L of hydrogen? Edit View Insert Format Tools Table 12pt v Paragraph v B IUAV2V ぴく O words レ7 …arrow_forward
- Pressures up to 2000 atm are measured with a dead-weight gauge. The piston diameter is 4 mm. What is the approximate mass in kg of the weights required? [Write your answer to the nearest whole number]arrow_forwardName: Peer Leader: Date: For all problems involving calculations, show your work. Carry the units through each calculation and follow significant figure rules. 1. atm 2.44 mmHg Module 7 Worksheet 452 Torr 960 kPa 237 PSI 20.7 2. There are 36.7 grams of ammonia gas trapped in a non- deformable container. Calculate the volume of the gas occupied in the container under STP conditions. 187 3. Calculate the volume of 5.9 x 1023 molecules of propane gas trapped in a container at a pressure of 253.3 kPa and a temperature of 269 °F 4. A lightbulb contains 0.0753 g of helium gas with a pressure of 189 torr at 22.0 °C. The light bulb is then turned on and, after a period of time, the temperature rises to 279 °C. Calculate the final pressure inside the lightbulb. 5. In a car engine, a 425 mL cylinder and piston system is filled with a mixture of 0.83 g and 0.37 g of octane and oxygen gas, respectively. Both gases are at a temperature of 28 °C. Ifarrow_forwardPlease send me the question in 20 minutes it's very urgent plzarrow_forward
- Borrowe NY Ellucian Degree Wor.. O Dayforce A ALEKS - Jada Jacks.. M MyOpenMath Question 8 Objective Knowledge Check Here is a graph of the probability of an atom moving with a particular speed, for a sample of argon gas at - 154. °C. probability 200 300 400 500 600 100 speed (m/s) Use this graph to answer the following questions. Round each of your answers to the nearest m/s. Note: your answers must be within 25 m/s of the exact answers to be graded correct. m What is the most likely speed of a Ar atom in this sample? m What higher speed is only half as likely as the most likely speed? m What higher speed is only 10% as likely as the most likely speed? I Don't Know Submit O 2021 McGraw-Hill Ed MacBook Pro SC Thank The $arrow_forwardA chemistry graduate student is designing a pressure vessel for an experiment. The vessel will contain gases at pressures up to 70.0 MPa. The student's design calls for an observation port on the side of the vessel (picture). The bolts that hold the cover of this port onto the vessel can safely withstand a force of 2.40 MN. Calculate the maximum safe diameter w of the port. Round your answer to the nearest 0.1cmarrow_forward- Part B The kinetic molecular model details other aspects of gas particle motion and collisions besides speed. Open the Run Experiment tool under the Experiment tab in the simulation, and then select the Submicroscopic tab. You can track the motion of an individual gas particle by clicking the Track button in the lower right corner, and you can track a different gas particles by first clicking Pause, then clicking on a gas particle, and finally clicking Resume Change several of the macroscopic conditions (temperature, pressure, and volume) of th over a consistent amount of time (e.g, 10 s). Then, complete the following statements that describe the atomic behavior of a gas particle. ideal gas and observe how the movement of a tracked gas particle is affected by those changes and collisions. For each condition, make your observations Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your…arrow_forward
- Assuming the initial gas pressure are equal, match the gas to the appropriate cylinder in the diagram. The three gases you can chose from are Cl₂, N₂, and CO₂. blue: CO2 red: N2 green: C12arrow_forwardPlease do all parts if possible, I dont need explanation just answer, Thank you: TRUE OR FALSE: a) Glasses are usually harder than plastics but softer than silicon carbide and boron carbide b) Any amorphous (lacking in long-range periodic order) materials, inorganic, organic, or metallic, formed by any techniques, exhibits glass transformation formation behavior are glasses c) In general, the viscosity is low at the melting position of glass, and high at room temperature. d) In general, if the viscosity is very high at the melting position of the corresponding crystalline phase which would form the melt, it is much easier to form glass for this melt. e) The glass fiber is possible to have a tensile strength even larger than that for iron or structural steel. Therefore, in the practical applications, we should be able to use glasses for the same purposes of the iron or structural steel.arrow_forwardHydraulic lifts work on the principle of:* - a) Archimedes’ principle - b) Pascal’s law - c) Newton’s third law - d) Bernoulli’s principlearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY