
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Show work with explanation needed...don't give Ai generated solution
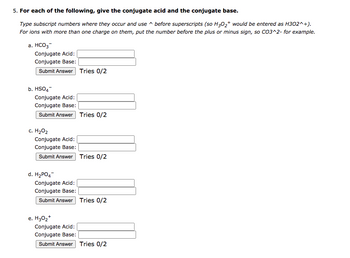
Transcribed Image Text:5. For each of the following, give the conjugate acid and the conjugate base.
Type subscript numbers where they occur and use
before superscripts (so H3O2+ would be entered as H302^+).
For ions with more than one charge on them, put the number before the plus or minus sign, so CO3^2- for example.
a. HCO3
Conjugate Acid:
Conjugate Base:
Submit Answer Tries 0/2
b. HSO4
Conjugate Acid:
Conjugate Base:
Submit Answer Tries 0/2
c. H₂O₂
Conjugate Acid:
Conjugate Base:
Submit Answer Tries 0/2
d. H2PO4
Conjugate Acid:
Conjugate Base:
Submit Answer Tries 0/2
e. H30₂+
Conjugate Acid:
Conjugate Base:
Submit Answer Tries 0/2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen atom? b. the electronegativity of the element bonded to the oxygen atom that bears the acidic hydrogen? c. the number of oxygen atoms? How does the strength of a conjugate base depend on these factors? What type of solution forms when a nonmetal oxide dissolves in water? Give an example of such an oxide. What type of solution forms when a metal oxide dissolves in water? Give an example of such an oxide.arrow_forwardNovocaine, C13H21O2N2Cl, is the salt of the base procaine and hydrochloric acid. The ionization constant for procaine is 7106. 15 a solution of novocaine acidic or basic? What are [H3O+], [OH-], and pH of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g/mL.arrow_forward. Consider 0.25 M solutions of the following salts: NaCl. RbOC1, KI, Ba(ClO4),, and NH4NO3. For each salt, indicate whether the solution is acidic, basic, or neutral.arrow_forward
- Using the diagrams shown in Problem 10-37, which of the four acids is the weakest acid?arrow_forwardWrite the formula and name for the conjugate acid of the following substances. (The information in Tables 15.6 and 15.8 may be helpful.) (a) N2H4 (b) NO2 (c) ClO4 (d) Iarrow_forwardAre solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced chemical equations for the reactions causing the solution to be acidic or basic. The relevant Ka and Kb values are found in Tables 13-2 and 13-3. a. NaNO3 b. NaNO2 c. C5H5NHClO4 d. NH4NO2 e. KOCl f. NH4OClarrow_forward
- Write a formula for the conjugate base formed when each of the following behaves as a Brnsted acid: a. HSO3 b. HPO42 c. HClO3 d. CH3NH3+ e. H2C2O4arrow_forwardIn the following net ionic reaction, identify each species as either a Brnsted-Lowry acid or a Brnsted -Lowry base: CH3COO(aq)+HS(aq)CH3COOH(aq)+S2(aq). Identify the conjugate of each reactant and state whether it is a conjugate acid or a conjugate base.arrow_forwardAre solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka, and Kb values are found in Tables 13-2 and 13-3. a. Sr(NO3)2 b. NH4C2H3O2 c. CH3NH3Cl d. C6H5NH3ClO2 e. NH4F f. CH3NH3CNarrow_forward
- . Strong buses are bases that completely ionize in water to produce hydroxide ion, OH-. The strong bases include the hydroxides of the Group I elements. For example, if 1.0 mole of NaOH is dissolved per liter, the concentration of OH ion is 1.0 M. Calculate the [OH-], pOH, and pH for each of the following strong base solutions. a. 1.10 M NaOH b. 2.0104M KOH c. 6.2103M CsOH d. 0.0001 M NaOHarrow_forwardTwo strategies are followed when solving for the pH of an acid in water. What is the strategy for calculating the pH of a strong acid in water? What major assumptions are made when solving strong acid problems? The best way to recognize strong acids is to memorize them. List the six common strong acids (the two not listed in the text are HBr and HI). Most acids, by contrast, are weak acids. When solving for the pH of a weak acid in water, you must have the Ka value. List two places in this text that provide Ka values for weak acids. You can utilize these tables to help you recognize weak acids. What is the strategy for calculating the pH of a weak acid in water? What assumptions are generally made? What is the 5% rule? If the 5% rule fails, how do you calculate the pH of a weak acid in water?arrow_forwardBoron trifluoride, BF3, and diethyl ether, (C2H5)2O, react to produce a compound with the formula BF3 : (C2H5)2O. A coordinate covalent bond is formed between the boron atom on BF3 and the oxygen atom on (C2H5)2O. Write the equation for this reaction, using Lewis electron-dot formulas. Label the Lewis acid and the Lewis base. Determine how many grams of BF3: (C2H5)2O are formed when 9.10 g BF3 and 23.3 g (C2H5)2O are placed in a reaction vessel, assuming that the reaction goes to completion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax