
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
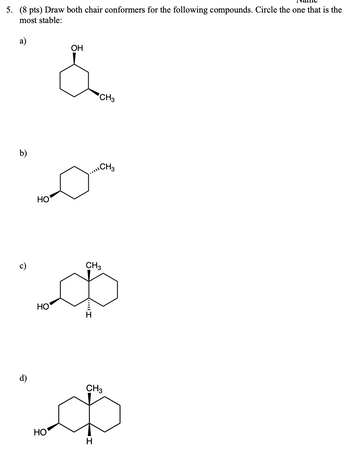
Transcribed Image Text:5. (8 pts) Draw both chair conformers for the following compounds. Circle the one that is the
most stable:
a)
OH
CH3
b)
CH3
HO
HO
CH3
ه
I
HO
CH3
Ф
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- myo-Inositol, one of the isomers of 1,2,3,4,5,6-hexahydroxycyclohexane, acts as a growth factor in both animals and microorganisms. Draw the most stable chair conformation of myo-inositol.arrow_forwardFollowing is a chair conformation of cyclohexane with the carbon atoms numbered 1 through 6. (a) Draw hydrogen atoms that are above the plane of the ring on carbons 1 and 2 and below the plane of the ring on carbon 4. (b) Which of these hydrogens are equatorial? Which are axial? (c) Draw the alternative chair conformation. Which hydrogens are equatorial? Which are axial? Which are above the plane of the ring? Which are below it?arrow_forwardGiven the structures of the following two tetramethylcyclohexanes: CH3 CH3 CH3 "CH3 CH3 CH3 A В (a) Draw the most stable chair conformation for both A and B. Which structure is lower in energy (most stable)? (b) Ring flip the above structures and draw the least stable chair conformation for both A and B. Which structure is highest in energy (least stable)?arrow_forward
- Draw the most stable chair conformation of the following compound: O OHarrow_forward1.3 Draw the most stable (briefly explain) chair conformation for compound A. Draw both conformations first and then identify the least stable conformer. но. 5 0 3 1 HO NH2 A 3arrow_forwardDraw a Newman projection for two more staggered conformations of this molecule. Which of your conformations is most stable? Assume that -OH and -CH3 are comparable in size.arrow_forward
- (5) a. Draw the LOWEST energy conformer of the following compound in a Newman Projection. The projection should be as if you are looking down the 2-1 C-C bond, with carbon 2 in front.arrow_forward1E.) Which of the following two conformers is lower in energy? Both conformers are of equal enery O A Conformer A is lower in energy. B Conformer B is lower in energy. It cannot be determinedarrow_forward3. Which of the following statements best explains the reason for the relative stabilities of the two conformers shown below? CH3 H3C CH3 H₂ CH3 H H H H H3C CH3 A. B. A) A has more torsional strain; B has more steric strain. B) A has more steric strain; B has more torsional strain. C) A and B have the same torsional strain. D) A and B have the same steric strain. E) A and B have similar steric strain; B has more torsional strain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning