
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
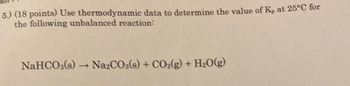
Transcribed Image Text:5.) (18 points) Use thermodynamic data to determine the value of Kp at 25°C for
the following unbalanced reaction:
NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
->
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Suppose you have an endothermic reaction with H = + 15 kJ and a S of 150 J/K. Calculate G and Keq at 10, 100, and 1000 K.arrow_forwardConsider the reaction NH4+(aq) H+(aq)+NH3(aq) Use G f for NH3(aq) at 25C=26.7 kJ/mol and the appropriate tables to calculate (a) G at 25C (b) Ka at 25Carrow_forwardThe equilibrium constant for a reaction decreases as temperature increases. Explain how this observation is used to determine the sign of either H or S.arrow_forward
- Consider a metal ion A2+ and its nitrate salt, In an experiment, 35.00 mL of a 0.217 M solution of A(NO3)2 is made to react with 25.00 mL of 0.195 M NaOH. A precipitate, A(OH)2, forms. Along with the precipitation, the temperature increases from 24.8C to 28.2C. What is H for the precipitation of A(OH)2? The following assumptions can be made. • The density of the solution is 1.00 g/mL. • Volumes are additive. • The specific heat of the solution is 4.18 J/g C.arrow_forwardCalculate K at 25°C for each of the reactions referred to in Question 32. Assume smallest whole-number coefficients.arrow_forwardSuppose you have an exothermic reaction with H = 15 kJ and a S of +150 J/K. Calculate G and Keq at 10, 100, and 1000 K.arrow_forward
- At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s), to chlorine and iodine gases. The partial pressure of chlorine gas at equilibrium is three times that of iodine gas. What are the partial pressures of iodine and chlorine at equilibrium?arrow_forward5.11. Determine the numerical value of Q for the reaction conditions indicated.arrow_forwardIs the following reaction spontaneous as written? Explain. Do whatever calculation is needed to answer the question. SO2(g)+H2(g)H2S(g)+O2(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning