
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
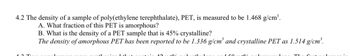
Transcribed Image Text:4.2 The density of a sample of poly(ethylene terephthalate), PET, is measured to be 1.468 g/cm³.
A. What fraction of this PET is amorphous?
B. What is the density of a PET sample that is 45% crystalline?
The density of amorphous PET has been reported to be 1.336 g/cm³ and crystalline PET as 1.514 g/cm³.
150
10
TL
Expert Solution
arrow_forward
Step 1

Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Orange peels, containing 20% oil, 30% water, and 50% insoluble matter, are extracted by contacting with fresh organic solvent hexane. Hexane and water are assumed immiscible. 90% of the oil is recovered in the solution (extract) containing 50% by mass of oil. The raffinate containing the unextracted oil has a mass of 55 kg. Calculate mass of orange peel that was processed (kg), mass of fresh hexane used (kg), concentration of oil in the raffinate stream (mass percent).arrow_forwardSteelworks activities are important sources of fine particles, which may affect air quality in urban areas close to Fe-Mn-alloy manufacturing plants. In order to identify the identity of the particles, fine particles were sampled inside the chimneys of the plant. Explain briefly the surface analytical techniques in order to identify: (i) Particle composition. (ii) Particle size. (ii) Fe and Mn oxidation states in fine particles.arrow_forwardWhat is the molarity of a solution prepared by dissolving 3.02 grams of NaOH (molar mass 40.0 g mol-1) in water to make 125 mL of solution?arrow_forward
- 1.6 Properties: Diffusivity Estimate the molecular diameter and diffusion coefficient for the proteins ribonuclease (MW 13,700 Da), hemoglobin (MW 68,000), and urease (MW 480,000), assuming the molecules are spherical and the density of each protein molecule is 1.3 g/cm³.arrow_forwardAn Al-4.5wt%Cu alloy has a low-temperature yield stress of 600 MPa due to the precipitation strengthening effect imparted by the equilibrium q phase (Al2Cu) precipitates. Estimate the interparticle spacing and particle size in this alloy. Given: density of α-solid solution 2,700 kg/m3 and density of q phase 4,430 kg/m3. Assume the Cu content in α-solid solution 0.5 wt% and the Cu content in the q phase 54%. Hint: You need to use the Orowan model of particle strengthening and the lever rule among other concepts.arrow_forwardnumber 2 ONLYarrow_forward
- The percent by mass of phenol (MM= 94.11 g/mol) in an aqueous solution is 10.9%. What is the molality of the phenol solution?arrow_forwardUsing the following data, compare the effect of supersaturation ratio over the range of 1.005 to 1.02 on the primary homogeneous nucleation of AgNO3, NaNO3, and KNO3 from aqueous solutions at 25oC:arrow_forwardClassify each of the following substances as either a strong or weak acid, strong or weak base, or a soluble or insoluble salt. Clear All strong acid HCN weak acid KCIO, strong base NH3 weak base NaOH soluble salt HF insoluble saltarrow_forward
- The standard molar enthalpy of formation of calcium carbonate (CaCO) is -1206.9 kJ/mol. What will be the enthalpy for the formation of 75 g of CaCO? (MM-100.1 g/mol) a.) -1206.9 kJ b.) -9043 kJ c.) 16.09 kJ d.) 603.4 kJarrow_forwardConsider Polyethylene, the polymer made from ethylene. Calculate the molar mass of a Polyethylene molecule that contains 5 x 104 C2H4 units.arrow_forward2. What would the coordination number and coordination geometry be for a crystalline ceramic with a cation-anion radius ratio of 0.285?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The