
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
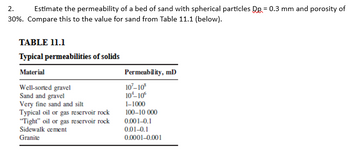
Transcribed Image Text:2.
Estimate the permeability of a bed of sand with spherical particles Qp = 0.3 mm and porosity of
30%. Compare this to the value for sand from Table 11.1 (below).
TABLE 11.1
Typical permeabilities of solids
Material
Well-sorted gravel
Sand and gravel
Very fine sand and silt
Typical oil or gas reservoir rock
"Tight" oil or gas reservoir rock
Sidewalk cement
Granite
Permeability, ml)
107-108
10¹-10°
1-1000
100-10 000
0.001-0.1
0.01-0.1
0.0001-0.001
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- Calculate the ionic packing factor of sodium chloride. Magnesium oxide has a sodium chloride crystal structure. Determine the lattice parameter for sodium chloride and magnesium oxide.arrow_forwardFor the absorption of solute A through a falling film, its Peclet number, Npe is in proportion to Select one or more: A. The average velocity of the falling film. B. All of the given choices. C. Diffusivity (DAB) D. Thickness of the falling film E. The reciprocal of diffusivity (1/DaB) O OOSOD OOSOLarrow_forwardAnswer 17.6!! 17.1 is listed for refearrow_forward
- A 40% (volume) suspension of spherical sand particles in an oil has a hindered settling velocity of 3.25 µm/s. If the Richardson-Zaki hindered settling index is 4.5 then, the terminal velocity of a sand grain is: a) 0.326 µm/s b) 1.25 µm/s c) 2 µm/s d) 0.8 µm/sarrow_forwardSketch two graphs, the first for a solid that's solubility is increasing as a function of temperature, and the second for a solid whose solubility decrease as a function of temperature. Be sure to label each axisarrow_forward1. Summary the classification of pores 2. Summary the factors affecting porosityarrow_forward
- 2. The true density of the solid material in an activated alumina particle is 3.675 g/cm3. The density of the particle determined by mercury displacement is 1.547 g/cm3. The surface area (Sg) by adsorption measurement is 175 m²/g. The bulk density of bed (which is defined as mass of catalyst particle per volume of reactor bed) is 0.81 g/cm³. From this information, Determine, a) the pore (void) volume per gram, b) the porosity of the particles, c) the mean pore radius (ā), d) what fraction of the total volume of the bed is void space between the particles, e) what fraction of the total volume of the bed is void within the particlesarrow_forwardin a 2-hour carburizing treatment, what temperature is required to obtain a 0.5% concentration of carbon at a depth of 0.5 mm below the surface of steel with a carbon concentration of 0.2%?considers that the inert gas used is enriched with carbon atoms at a concentration of 1.10% and that iron has an fcc structurearrow_forward1.a)What is the effect of surface roughness on interfacial strength? How does it depend on wettability? b)Why does chemical bonding typically result in stronger interfaces compared to mechanical bonding?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The