
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please try to give type solution fast in 5 mins. will rate for sure
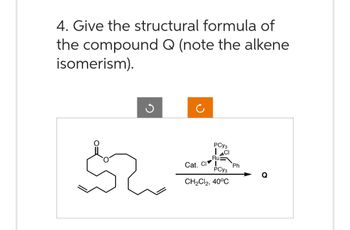
Transcribed Image Text:4. Give the structural formula of
the compound Q (note the alkene
isomerism).
J
PCY3
Ru
Cat. CI
PCY3
CH₂Cl2, 40°C
Ph
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The glassware which contacts the KMnO4 solution (the filter, suction flask and storage bottle), should all be pre-rinsed with a 10% HCl solution, AND THEN FULLY RINSED WITH DISTILLED WATER (VERY IMPORTANT STEP!!! – WHY??arrow_forward© Macmillan Learning The concentration of arsenic trioxide (As2O3) can be experimentally determined via titration with coulometrically generated iodine. To perform the analysis, solid As2O3 (MW = 197.84 g/mol) is first dissolved in an aqueous sodium bicarbonate solution, forming arsenious acid (As(OH)3) by the equilibrium shown. As2O3(s) + 3H2O(1) 2 As(OH)3(aq) The iodine is coulometrically generated by passing a constant current through the solution which contains potassium iodide (KI ). The arsenious acid in solution is then oxidized by the iodine. Once the reaction has gone to completion, excess generated iodine reacts with a starch indicator, generating a color change and signaling the titration end point. The amount of time it takes to reach the end point is used to determine the amount of As2O3 in solution. 21¯¯ = 12 + 2e¯ 12 + As(OH)3 + H2O = AsO(OH)3 + 2H+ + 21¯ An unknown amount of As2O3 was dissolved in 53.00 mL of an aqueous sodium bicarbonate solution, and to this sample,…arrow_forwardAnswer ASAParrow_forward
- 20 E The volume of water needed to dissolve 0.0620 grams of copper(II) carbonate is Assume no volume change upon addition of the solid. Submit Answer $ 4 R % 5 [Review Topics) References) Use the References to access important values if needed for this question. Retry Entire Group 8 more group attempts remaining Cengage Learning Cengage Technical Support T A 6 MacBook Pro Y & U ▶II * 00 8 1 ( 9 ) 0 P IL. Previous Email Instructorarrow_forwardQuantity Your Data 1. Grams of vinegar sample used for your titration 25.000 g 2. Initial Buret Reading of Sodium Hydroxide solution 10.00 mL 3. Final Buret Reading of Sodium Hydroxide solution 28.00 mL 4. Amount of Sodium Hydroxide Solution used to neutralize the vinegar sample 5. Concentration of NaOH in the NaOH solution 0.050 g/mL 6. Grams of NaOH used to neutralize the vinegar 7. Grams of acetic neutralized by the amount of NaOH 8. Percent acetic acid in the vinegar Hints: For #4, The amount of NaOH used is the difference between the starting buret value and the ending buret value. For #6, Once you calculate the amount of sodium hydroxide used, multiple that value by the concentration of NaOH in the NaOH solution. For #7, Multiply the value obtained in number 6 by the number 1.5. Remember, we learned that every 1 gram of NaOH neutralizes 1.5 grams of acetic acid. For #8, Divide the grams of acetic acid by the grams of vinegar sample and multiply this value by…arrow_forwardin text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forward
- in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forwardTrial 1 Trial 2 Trial 3 Initial burette reading (mL) 2.29 1.41 1.95 Molarity of NaOH (M) 0.100 0.100 0.100 Volume of vinegar sample (mL) 5.00 5.00 5.00 Final burette reading (mL) 50.37 49.39 49.84 Table 2. Titration data Trial 1 Trial 2 Trial 3 Initial burette reading (mL) 2.29 1.41 1.95 Molarity of NaOH (M) 0.100 0.100 0.100 Volume of vinegar sample (mL) 5.00 5.00 5.00 Final burette reading (mL) 50.37 49.39 49.84 Expected color at end point Volume of NaOH used (mL) 48.08 47.98 47.89 Compute for the ff: a. Average moles of acetic acid (mol)? b. Average molarity of acetic acid (M)? c. Average molarity of acetic acid (M)?arrow_forwardResults and Conclusion First Paragraph: First sentence matches with the first sentence of the Objective First sentence is: to identify the given ammonium salt and to determine determine its equivalent mass via acid-base titration Please calculate Average molar mass, % relative range, error, Identity of Unknown stated Second Paragraph: sources of errors discussed Please please please answer super super fast it's super importantarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY