Using the image attached for conclusion please make 1 paragraph - The cations present in cation mixture were... - The anions present in anions mixture were... - The cations present in unknown solution were... - The anions present in unknown solution were... Please please please answer as fast as possibl
Using the image attached for conclusion please make 1 paragraph - The cations present in cation mixture were... - The anions present in anions mixture were... - The cations present in unknown solution were... - The anions present in unknown solution were... Please please please answer as fast as possibl
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 3P
Related questions
Question
Using the image attached for conclusion please make 1 paragraph
- The cations present in cation mixture were...
- The anions present in anions mixture were...
- The cations present in unknown solution were...
- The anions present in unknown solution were...
Please please please answer as fast as possible please
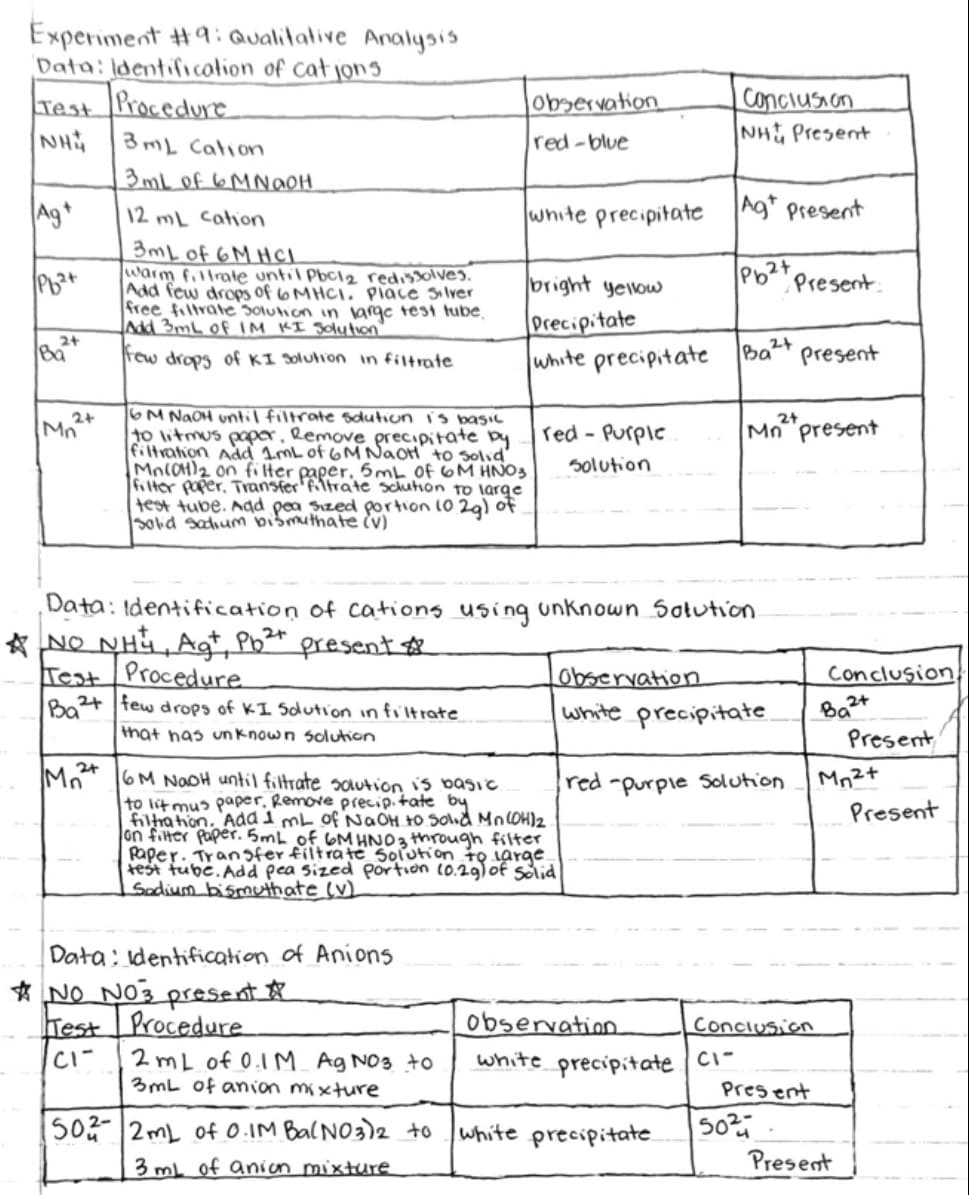
Transcribed Image Text:Experiment #9: Qualitative Analysis
Data: Identification of cations
Test Procedure
NH
Ag+
Pb2+
3mL Cation
3mL of 6MNaOH
12 mL cation
observation
red-blue
2+
2+
Mn
3mL of 6MHCL
warm filtrate until Pbcl2 redissolves.
Add few drops of 6MHCI. Place Silver
free filtrate Solution in large test tube.
Add 3mL of IM KI Solution"
few drops of KI Solution in filtrate
6M NaOH until filtrate solution is basic
to litmus paper. Remove precipitate by
filtration Add 1mL of 6M NaOH to solid
Mn(OH)2 on filter paper, 5mL of 6M HNO3
filter paper, Transfer filtrate solution to large
test tube. Add pea sized portion 10.2g) of
solid sodium bismuthate (V)
Conclusion
NH Present
white precipitate
Agt Present
bright yellow
Pb2+ Present
Bapresent
2+
Mn present
Precipitate
white precipitate
red - Purple
Solution
Data: Identification of cations using unknown Solution.
NO NH4, Agt, Pb2+ present &
Test
Procedure
Ba2+ few drops of KI Solution in filtrate
that has unknown solution
M+ 6M NaOH until filtrate solution is basic
to litmus paper. Remove precipitate by
filtration. Add 1 mL of NaOH to Solid Mn(OH)2
on filter paper. 5mL of 6M HNO3 through filter
Paper. Transfer filtrate Solution to large.
test tube. Add pea sized portion (0.2g) of Solid
Sodium bismuthate (v)
Data: Identification of Anions
NO NO3 present
Test Procedure
CI- 2mL of 0.1M AgNO3 to
3mL of anion mixture
Observation
white precipitate.
Conclusion
B92+
Present
red-purple Solution.
Mn2+
Present
Conclusion
observation
white precipitate CI-
5022mL of 0.1M Ba(NO3)2 to
3 mL of anion mixture
Present
white precipitate
502
Present
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 30 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning