
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Results and Conclusion
First Paragraph: First sentence matches with the first sentence of the Objective
First sentence is: to identify the given ammonium salt and to determine determine
its equivalent mass via acid-base titration
Please calculate Average molar mass, % relative range, error, Identity of Unknown stated
Second Paragraph: sources of errors discussed
Please please please answer super super fast it's super important
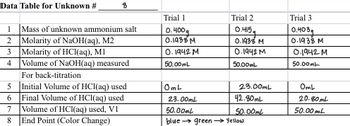
Transcribed Image Text:Data Table for Unknown #
8
1 Mass of unknown ammonium salt
2 Molarity of NaOH(aq), M2
3 Molarity of HCl(aq), M1
4 Volume of NaOH(aq) measured
For back-titration
5 Initial Volume of HCl(aq) used
Final Volume of HCl(aq) used
6
7
Volume of HCl(aq) used, V1
End Point (Color Change)
8
Trial 1
0.400q
0.1938 M
0.1942 M
50.00mL
OmL
Trial 2
0.415a
0.1938 M
0.1942 M
50.00mL
23.00mL
42.80mL
50.00ml
23.00mL
50.00mL
blue green → Yellow
Trial 3
0.403g
0.1938 M
0.1942 M
50.00mL
OmL
20.80mL
50.00mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Just as pH is the negative logarithm of H3O+], pKa is the negative logarithm of Ka, pKa = – log Ka The Henderson-Hasselbalch equation is used to calculate the pH of buffer solutions: pH = pK, +log base] [acid] %3D Notice that the pH of a buffer has a value close to the pKa of the acid, differing only by the logarithm of the concentration ratio [base]/[acid].arrow_forwardThe "buffer region" of a titration curve occursa) the intial solution titrated is a buffer.b) post-equivalencec) pre-equivalenced) at equivalencearrow_forward(Incorrect) 367 mL of a 0.64 M hydrochloric acid solution is added to 238 mL of a 0.1 M hydroiodic acid solution. Calculate the pOH of the resulting solution. 0.37 (Your answer) 0.13 0.43 13.63 (Correct answer) 13.87arrow_forward
- Use the References to access important values if needed for this question. An aqueous solution contains 0.367 M hypochlorous acid. How many mL of 0.224 M potassium hydroxide would have to be added to 125 mL of this solution in order to prepare a buffer with a pH of 7.300? Submit Answer Retry Entire Group mL 8 more group attempts remaining Pravious Email Instructor Next> Save and Exitarrow_forwardThe experimental procedure above was used to generate the data in the tables below. Data Collection Table Titration Mass of Sample Initial Buret Reading (mL) Final Buret Reading (mL) 1 1.234 1.05 19.95 2 1.213 4.00 23.50 3 1.273 0.30 20.05 4 1.292 5.95 25.65 - For each titration, calculate: - The number of moles of potassium permanganate used in the titration (H x L of KMnO4) - The number of moles of iron present in the sample (stoichiometry in balanced equation) - The percentage by mass of iron in the solid sample (Equation 1) - The average mass % of iron in your unknown - The standard deviation in your % Fe results:arrow_forwardPlase don't provide handwritten solutionarrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward9.4 mL of 2.4 M HCI is used to titrate 12.6 mL of NH3 its endpoint. What is the initial molar concentration of the NH3? answer to two significant figures.arrow_forwardPlease correct answer and don't use hend raitingarrow_forward
- Describe the information necessary (and why) to choose a good indicator for a weak acid/strong base titration. Answer:arrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. An aqueous solution contains 0.370 M acetic acid. How many mL of 0.361 M sodium hydroxide would have to be added to 150 mL of this solution in order to prepare a buffer with a pH of 4.400? Submit Answer Retry Entire Group mL 7 more group attempts remaining Previous Email Instructor Naxt Save and Exitarrow_forwardSolve correctly please. (Gpt/Ai wrong answer not allowed)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY