
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
Can you solve this and explain why
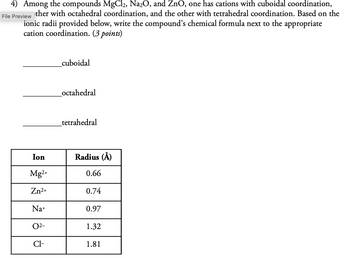
Transcribed Image Text:4) Among the compounds MgCl2, Na2O, and ZnO, one has cations with cuboidal coordination,
File Preview ther with octahedral coordination, and the other with tetrahedral coordination. Based on the
ionic radii provided below, write the compound's chemical formula next to the appropriate
cation coordination. (3 points)
_cuboidal
_octahedral
tetrahedral
Ion
Radius (Å)
Mg2+
0.66
Zn2+
0.74
Na+
0.97
02-
1.32
Cl-
1.81
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Help me pleasearrow_forwardProblem #4 a) Find the indices for the directions indicated by the two vectors in the shown sketch below 0.5 mm 0.4 mm 0.7 mm Direction 1 Direction 2 ty b) Sketch the following directions within a cubic unit cell: (a) [123], (b) [211], (c) [102], (d) [133]. c) Sketch the following planes within a cubic unit cell: (a) (011), (b) (102), (c) (112), and (d) (131)arrow_forwardWhat is the angle between the [102] and [111] directions in the cubic system? Select one: a. 65,1 O b. 19.47° O c. 31.4° O d. 39.23° Rhodium (Rh) has the Face-Centered Cubic (FCC) crystal structure. The atomic radius of Rhodium is r= 0.134 nm (20°C). Calculate the edge length "a" of the unit cllarrow_forward
- Help me pleasearrow_forwardCalculate the theoretical density of NiO, given that it has the rock salt crystal structure. You may want to use the table below. The atomic weights for Ni and O are 58.69 g/mol and 16.00 g/mol, respectively. Ionic Radius (nm) P = i Cation Fe²+ Ni²+ Mg2+ Mn2+ g/cm³ 0.077 0.069 0.072 0.067 Anion 0²- S²- Ionic Radius (nm) 0.140 0.184arrow_forwardA number of elements along with their crystal structures and atomic radii are listed in the following table. Which pairs might be expected to have complete solid solubility in each other? Crystal Atomic Crystal Structure Atomic Structure radius (nm) radius (nm) Silver Palladium FCC 0.144 Lead FCC 0.175 FCC 0.137 0.137 Tungsten Rhodium ВСС Copper Gold FCC 0.128 FCC 0.134 Platinum Tantalum FCC 0.144 FCC 0.138 Nickel FCC 0.125 ВСС 0.143 Aluminum Sodium FCC 0.143 Potassium ВСС 0.231 ВСС 0.185 Molybdenum ВСС 0.136arrow_forward
- Convert the following Miller indices to directions and sketch the directions within a cubic unit cell: (a) [301] (b) [301] (c) [301] (d) [122] (e) [122] [122] (g) [111] (h) [T11] (1) [171] V) [117] Question 2: The change of SO, concentration with time is shown in Table 1. t(s) [C] 1.000000 100 0.778801 200 0.606531 300 0.472367 400 0.367879 500 0.286505 600 0.223130 800 0.135335 1000 0.082085 1200 0.049787 The process is governed by the rate law given by the equation: In(SO)-k1+ In (SO), Where k is the rate constant and is the time, and In is natural logarithm. By calculating the corresponding values of In [SO,] and plotting the graph of In [SO,] against time, r, determine the rate constant k of the reaction.arrow_forwardPlease don't provide handwriting solutionarrow_forwardThe plot below shows bonding energy vs. interatomic separation for two elements. АВ a A. Which element would you expect to have a lower melting point? A B same can't tell provide a brief justification of your choice. B. Which element would you expect to have a smaller lattice constant? A В same can't tell provide a brief justification of your choice. C. Which element would you expect to have a larger elastic modulus? A justification of your choice. В same can't tell provide a brief can't tell provide a brief D. Which element would you expect to have the highest yield stress? justification of your choice. A В same Bonding energyarrow_forward
- b. Given that Na = 6.022x1023 atoms/mol. Calculate the theoretical density of nickel. Show your calculations.arrow_forwardSolve this and show all of the calculationsarrow_forward4. Indices of direction. If [hkl] are indices of a set of directions relative to lattice vectors a, a, az and [h'k'l'] are the indices of same direction relative to lattice vectors af, as, a, obtain the relation between two sets of indices. Hence show that, if aj, as, az are lattice vectors for the conventional hexagonal unit cell and a, as, a refer to the orthorhobic cell of an hcp structure, then h' k' 0 0 1 200 110 80.0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY