
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
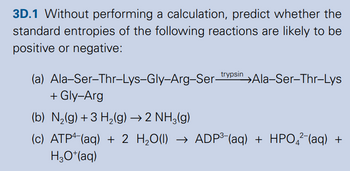
Transcribed Image Text:3D.1 Without performing a calculation, predict whether the
standard entropies of the following reactions are likely to be
positive or negative:
(a) Ala-Ser-Thr-Lys-Gly-Arg-Ser-trypsin →Ala-Ser-Thr-Lys
+ Gly-Arg
(b) N2(g) +3 H2(g) → 2 NH3(g)
(c) ATP (aq) + 2 H₂O(l) → ADP³- (aq) + HPO²¯(aq) +
H3O+(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- What is the stereochemical result of the Stecker synthesis of the amino acid alanine?arrow_forwardQuestion attachedarrow_forwardThe activity of an enzyme requires a glutamic acid to display its -COOH functional group in the protonated state. Suppose the pK, of the -COOH group is 4.07. (a) Will the enzyme be more active at pH 3.5 or 4.5? Explain. (b) What fraction of the enzymes will be active at pH = 4.07? Explain. (c) At what pH will the enzyme show 78% of maximal activity?arrow_forward
- Write out a scheme for the resolution of the two enantiomers of the antiplatelet drug clopidogrel with 10-camphorsulfonic acid.arrow_forwardWithout performing a calculation, predict whether the standard entropies of the following reactions are likely to be positive or negative:(a) Ala-Ser-Thr-Lys-Giy-Arg-Ser (tryspin) → Ala-Ser-Thr-Lys + Giy-Arg(b) N2(g) + 3 H2(g) → 2 NH3(g)(c) ATP4-(aq) + 2 H2O(l) → ADP3-(aq) + HPO4 2-(aq) + H3O+(aq)arrow_forwardPlease answer with positive or negative please in the blanks.arrow_forward
- The overall adenylate kinase reaction (2 ADP = AMP + ATP) is a combination of 3 reactions: ATP hydrolysis, pyrophosphate hydrolysis, and ATP formation. Show each step and provide the standard free energy (ΔG°′) for each and the overall reaction. If the adenylate kinase reaction is at equilibrium and intracellular [ATP] = 5 mM and [ADP] = 0.5 mM, calculate the concentration of AMP at pH 7 and 25 °C. Show all work. ATP (ADP+P) = -30.5 kj/mol; ATP (AMP+PP) = -45.6 kj/mol; PP = -19.2 kj/molarrow_forwardb) The methyl group at the C terminus of aspartame can be cleaved by concentrated sulfuric acid (conc. H₂SO4) to reveal the carboxylic acid (compound 1), which has a pK₂ of 2.43. OH NH₂ OCH3 conc. H₂SO4 OH NH₂ OH Compound 1 The other two pKa values in the molecule are unchanged. It is useful to calculate the isoelectric point (pl) when planning to separate charged species from neutral species with electrophoresis. pKal + PK₂2 2 pl = HINT: i) Calculate the isoelectric point of aspartame. ii) Calculate the isoelectric point of compound 1 and explain your reasoning behind the calculation.arrow_forwardWhich of the following combination in each pair is likely to produce more Maillard browning when heated at 95 °C for 4 hours? Explain the chemical basis for your choice. Sucrose + glycine, pH 7.0 vs. glucose + glycine, pH 7.0 Maltose with a dextrose equivalency (DE) of 20 + glycine, pH 9.0 vs. maltose + glycine, pH 9.0 Lactose + glycine, pH 8.0 vs. lactose + glycine, pH 4.0arrow_forward
- Question attachedarrow_forwardP35.27 Bacteriorhodopsin is a protein found in Halobacterium halobium that converts light energy into a transmembrane proton gradient that is used for ATP synthe- sis. After light is absorbed by the protein, the following initial reaction sequence occurs: ic k1=2.0× 1012 s-1 Br k2=3.3 × 101' s-1 J - → K a. At what time will the maximum concentration of the inter- mediate J occur? ry b. Construct plots of the concentration of each species ver- sus time.arrow_forwardAn enzyme catalyzes the hydrolysis of an ester with a certain activity, but this activity is lost in a 3 M urea solution. What is the most likely explanation for the loss of activity? (A) Urea binds to the active site of the enzyme competitively with the substrate. (B) Urea causes the cleavage of the peptide bonds in the enzyme. (C) Urea causes the enzyme to denature and lose its specific three-dimensional shape. (D) Urea reacts with disulfide bonds in the enzyme.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
