
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
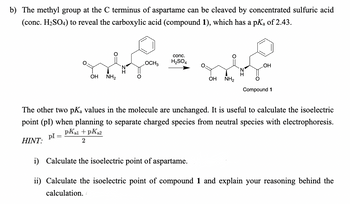
Transcribed Image Text:b) The methyl group at the C terminus of aspartame can be cleaved by concentrated sulfuric acid
(conc. H₂SO4) to reveal the carboxylic acid (compound 1), which has a pK₂ of 2.43.
OH NH₂
OCH3
conc.
H₂SO4
OH NH₂
OH
Compound 1
The other two pKa values in the molecule are unchanged. It is useful to calculate the isoelectric
point (pl) when planning to separate charged species from neutral species with electrophoresis.
pKal + PK₂2
2
pl =
HINT:
i) Calculate the isoelectric point of aspartame.
ii) Calculate the isoelectric point of compound 1 and explain your reasoning behind the
calculation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write out a scheme for the resolution of the two enantiomers of the antiplatelet drug clopidogrel with 10-camphorsulfonic acid.arrow_forwardAn enzymatic assay was performed using the enzyme phophofructokinase-1, which catalyses the committed step of the glycolysis pathway. What is the final concentration of the fructose 6-phosphate substrate in a test tube containing the following: 250 µL of 150pM fructose 6-phophate, 100 µL 20 mg.ml-1 phosphofructoskinase-1 and 150 µL 100mM buffer pH 7.5arrow_forwardDipalmitoylphosphatidylcholine is a surfactant found in the lining of the lungs. It prevents the lungs from collapsing when the lung volume is low and protects the lungs from injuries caused by inhaled particles. Draw its structure.arrow_forward
- Guanidine and the guanidino group present in arginine are two of the strongest organic bases known. Account for their basicity.arrow_forwardTrypsin is a serine protease that has a KM of 15 mM. At an initial substrate concentration of 250 µM insulin, trypsin is found to degrade insulin at a rate of 0.6 µM/min. If the concentration of trypsin in solution was 4.326 µM, what is the kcat of trypsin in s-1?arrow_forwardA biochemist completely digests a glycerophospholipid with a mixture of phospholipases A and D. HPLC and mass spectrometry analysis reveals the presence of an amino acid of 105,09 Da, a saturated fatty acid of 256.43 Da, and an omega-3 monounsaturated fatty acid of 282.45 Da. Which amino acid does the glycerophospholipid contain? O valine (C,H, NO,) O alanine (C, H,NO,) O scrine (C,H,NO,) O proline (C,H,NO,) Modify the phosphoglycerol backbone to draw the most likely structure of the lipid. Add the amino acid using the groups tool. Draw Rings Groups More Erase Selectarrow_forward
- Glutamic acid has three protonatable groups (two carboxyls and one amino). Calculate its isoelectric point, indicating what values of pKa you used to calculate it.arrow_forwardThe monoanion of adenosine monophosphate (AMP) is an intermediate in phosphate metabolism: А—О—р—он АMP—ОН- where A = adenosine. If the pKa for this anion is 7.21, what is the ratio of [AMP-OH ] to [AMP-02 ]in blood at pH 7.4?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY