
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
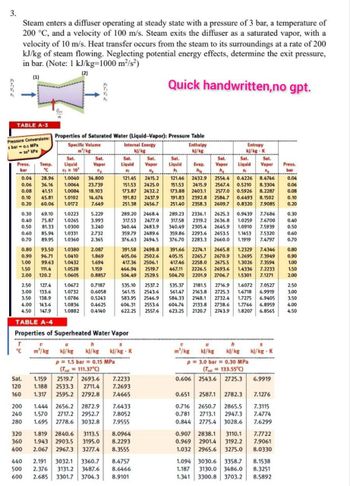
Transcribed Image Text:3.
Steam enters a diffuser operating at steady state with a pressure of 3 bar, a temperature of
200 °C, and a velocity of 100 m/s. Steam exits the diffuser as a saturated vapor, with a
velocity of 10 m/s. Heat transfer occurs from the steam to its surroundings at a rate of 200
kJ/kg of steam flowing. Neglecting potential energy effects, determine the exit pressure,
in bar. (Note: 1 kJ/kg-1000 m²/s²)
(1)
(2)
Quick handwritten,no gpt.
TABLE A-3
Qe
Pressure Conversions: Properties of Saturated Water (Liquid-Vapor): Pressure Table
1 bar -0.1 MPa
Specific Volume
m/kg
Internal Energy
10 kPa
kj/kg
Enthalpy
kj/kg
Entropy
kj/kg K
Sat.
Sat.
Sat.
Press.
bar
0.04 28.96
0.06 36.16
Temp.
"C
Liquid
Vapor
Liquid
Sat.
Vapor
Sat.
By x10
Evap. Vapor
he
1.0040 34.800
1.0064 23.739
0.08 41.51
1.0084 18.103
0.10
45.81
1.0102
14.674
0.20
60.06
1.0172
7.649
121.45 2415.2
151.53 2425.0
173.87 2432.2
191.82 2437.9
251.38 2456.7
Sat.
Liquid
hi
his
121.46 2432.9 2554.4 0.4226 8.4746
151.53 2415.9 2567.4 0.5210 8.3304
173.88 2403.1 2577.0 0.5926 8.2287
191.83 2392.8 2584.7 0.6493 8.1502
251.40 2358.3 2609.7 0.8320 7.9085
Sat.
Liquid
St
Sat.
Vapor
Press.
bar
0.04
0.06
0.08
0.10
0.20
0.30
69.10
1.0223
5.229
0.40
75.87
1.0265
3.993
0.50
81.33
1.0300
3.240
0.60 85.94
1.0331
2.732
0.70
89.95
1.0360
2.365
289.20 2468.4
317.53 2477.0
340.44 2483.9
359.79 2489.6
376.63 2494.5
289.23
2336.1
317.58 2319.2
340.49 2305.4
359.86 2293.6
376.70 2283.3
2625.3 0.9439
7.7686
0.30
2636.8 1.0259 7.6700
2645.9 1.0910 7.5939
2653.5 1.1453 7.5320
2660.0 1.1919 7.4797
0.40
0.50
0.60
0.70
0.80
93.50
1.0380
2.087
391.58 2498.8
0.90
96.71
1.0410
1.869
405.06 2502.6
1.00
99.63
1.0432
1.694
417.36 2506.1
1.50
111.4
1.0528
1.159
466.94 2519.7
2.00
120.2
1.0605
0.8857
2.50 127.4
1.0672
3.00 133.6
1.0732
0.7187
0.6058
3.50 138.9
1.0786
0.5243
4.00 143.6
1.0836
0.4625
4.50 147.9
1.0882 0.4140
504.49 2529.5
535.10 2537.2 535.37 2181.5 2716.9 1.6072 7.0527
561.15 2543.6 561.47 2163.8 2725.3 1.6718 6.9919
583.95 2546.9 584.33 2148.1 2732.4 1.7275 6.9405
604.31 2553.6 604.74 2133.8 2738.6 1.7766 6.8959
622.25 2557.6 623.25 2120.7 2743.9 1.8207 6.8565
391.66 2274.1 2665.8 1.2329
405.15 2265.7 2670.9 1.2695 7.3949
417.46 2258.0 2675.5 1.3026 7.3594
467.11 2226.5 2693.6 1.4336 7.2233
504.70 2201.9 2706.7 1.5301 7.1271
7.4346
0.80
0.90
1.00
1.50
2.00
2.50
3.00
3.50
4.00
4.50
TABLE A-4
Properties of Superheated Water Vapor
T
°C
m³/kg
kJ/kg
h
kJ/kg
kJ/kg - K
m³/kg
kJ/kg
h
kj/kg
kj/kg K
p = 1.5 bar =0.15 MPa
Sat.
1.159
(Tsat = 111.37°C)
2519.7 2693.6
7.2233
p = 3.0 bar = 0.30 MPa
(Tsat = 133.55°C)
0.606 2543.6 2725.3
6.9919
120
160
1.188 2533.3 2711.4
1.317 2595.2 2792.8
7.2693
7.4665
200
1.444 2656.2 2872.9
7.6433
0.651 2587.1 2782.3 7.1276
0.716
240 1.570 2717.2 2952.7
280 1.695 2778.6 3032.8
7.8052
7.9555
2650.7 2865.5 7.3115
0.781 2713.1 2947.3 7.4774
0.844 2775.4 3028.6
7.6299
320
360
1.819 2840.6 3113.5
1.943 2903.5 3195.0
400 2.067 2967.3 3277.4
8.0964
8.2293
8.3555
0.907 2838.1 3110.1
0.969 2901.4 3192.2
1.032 2965.6 3275.0
7.7722
7.9061
8.0330
440 2.191 3032.1 3360.7 8.4757
500 2.376 3131.2 3487.6
600 2.685 3301.7 3704.3
8.6466
1.187
8.9101
1.341
1.094 3030.6 3358.7 8.1538
3130.0 3486.0 8.3251
3300.8 3703.2 8.5892
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- T-3arrow_forwardSteam enters a turbine operating at steady state at 800°F and 450 Ibf/in2 and leaves as a saturated vapor at 0.8 Ibf/in2. The turbine develops 12,000 hp, and heat transfer from the turbine to the surroundings occurs at a rate of 2 x 106 Btu/h. Neglect kinetic and potential energy changes from inlet to exit. Determine the exit temperature, in °F, and the volumetric flow rate of the steam at the inlet, in ft³/s.arrow_forwardSteam enters a turbine operating at steady state at 440°C and 30 bar and leaves as a saturated vapor at 0.08 bar. The turbine develops 9000 kW, and heat transfer from the turbine to the surroundings occurs at a rate of 590 kW. Neglect kinetic and potential energy changes from inlet to exit. a. Determine the exit temperature, in °C. b. Determine the volumetric flow rate of the steam at the inlet, in m³/s. T₁-440°C P₁=30 bar Qout 590 kW 2 X₂ 100%(sat.vapor) P₂=0.08 bar W turbine = 9000 kWarrow_forward
- Steam enters a turbine operating at steady state at 850°F and 450 Ibf/in? and leaves as a saturated vapor at 1.0 lbf/in?. The turbine develops 12,000 hp, and heat transfer from the turbine to the surroundings occurs at a rate of 2x 106 Btu/h. Neglect kinetic and potential energy changes from inlet to exit. Determine the exit temperature, in °F, and the volumetric flow rate of the steam at the inlet, in ft/s. Step 1 Determine the exit temperature, in °F. T2 = °F.arrow_forwardSteam enters a turbine operating at steady state at 750°F and 450 lbf/in² and leaves as a saturated vapor at 0.8 lbf/in². The turbine develops 12,000 hp, and heat transfer from the turbine to the surroundings occurs at a rate of 2 x 106 Btu/h. Neglect kinetic and potential energy changes from inlet to exit. Determine the exit temperature, in °F, and the volumetric flow rate of the steam at the inlet, in ft3/s. Step 1 Your answer is correct. Determine the exit temperature, in °F. T2 = 94.3 Hint Step 2 °F. Determine the volumetric flow rate of the steam at the inlet, in ft³/s. (AV) 1 = i ft³/s Attempts: 1 of 4 usedarrow_forwardRefrigerant 134a enters an insulated diffuser as a saturated vapor at 120°F with a velocity of 1200 ft/s. The inlet area is 1.4 in?. At the exit, the pressure is 400 Ibf/in? and the velocity is negligible. The diffuser operates at steady state and potential energy effects can be neglected. Determine the mass flow rate, in Ib/s, and the exit temperature, in °F.arrow_forward
- Refrigerant 134a enters an insulated diffuser as a saturated vapor at 80°F with a velocity of 1400 ft/s. The inlet area is 1.4 in². At the exit, the pressure is 400 lb/in² and the velocity is negligible. The diffuser operates at steady state and potential energy effects can be neglected. Determine the mass flow rate, in lb/s, and the exit temperature, in °F. Step 1 Determine the mass flow rate, in lb/s. Your answer is correct. m = 28.887 Hint Step 2 lb/s. * Your answer is incorrect. Determine the exit temperature, in °F. T₂=276.41 °F Attempts: 1 of 4 usedarrow_forwardRefrigerant 134a enters a well-insulated nozzle at 200 lbf/in.2, 140°F, with a velocity of 120 ft/s and exits at 90 lbf/in.2 with a velocity of 1500 ft/s.For steady-state operation, and neglecting potential energy effects, determine the temperature, in °F, and the quality of the refrigerant at the exit.arrow_forwardI need some help in how to solve this problem. Any help will be appreciated. Thanksarrow_forward
- Refrigerant 134a enters a well-insulated nozzle at 200 lbf/in.2, 200°F, with a velocity of 120 ft/s and exits at 50 lbf/in.2 with a velocity of 1500 ft/s. For steady-state operation, and neglecting potential energy effects, determine the temperature, in °F, and the quality of the refrigerant at the exit.arrow_forward6.arrow_forwardSteam enters a turbine operating at steady state at 850oF and 450 lbf/in2 and leaves as a saturated vapor at 1.2 lbf/in2. The turbine develops 12,000 hp, and heat transfer from the turbine to the surroundings occurs at a rate of 2 x 106 Btu/h. Neglect kinetic and potential energy changes from inlet to exit. Determine the exit temperature, in oF, and the volumetric flow rate of the steam at the inlet, in ft3/s.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY