
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
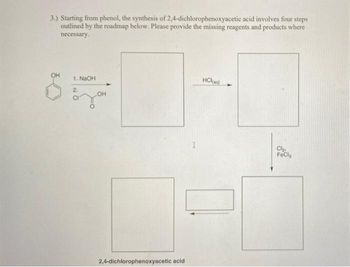
Transcribed Image Text:3.) Starting from phenol, the synthesis of 2,4-dichlorophenoxyacetic acid involves four steps
outlined by the roadmap below. Please provide the missing reagents and products where
necessary.
OH
1. NaOH
HCl(aq)
2
Cl₂
FeCla
OH
2,4-dichlorophenoxyacetic acid
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- which two of the following will react with weak base; sodium bicarbonate and which two will react with strong base; NaOH and which two will remain neutral. Write chemical reactions as well. COOH benzoic acid MP 122°C COOH o-toluic acid MP 101°C g 2-naphthol MP 123°C OH OH C(CH3)3 p-tert-butylphenol MP 101°C денз OCH3 p-dimethoxybenzene MP 57°C naphthalene MP 80°Carrow_forwardGive detailed Solution with explanation needed..don't give Handwritten answerarrow_forwardReaction of 1-butanol with periodane gives Butanal Propanoic acid 2-Butanone Butanoic acid as the major product.arrow_forward
- 16-57 Starting with either benzene or toluene, how would you synthesize the following substances? Assume that ortho and para isomers can be separated. (a) 2-Bromo-4-nitrotoluene (c) 2,4,6-Tribromoaniline (b) 1,3,5-Trinitrobenzene (d) m-Fluorobenzoic acidarrow_forwardH₂C ཏཱཏི 1 ནི OH 1. Br2, PBг3 2. H₂O H3C OH Br The a-bromination of carbonyl compounds by Br2 in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br2 reacts in an a-substitution reaction. Hydrolysis of the acid bromide completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions :0: H3C Br Br + :::OH2 Br H₂O H3C Br заarrow_forwardWhich combination of starting compounds should be chosen to prepare pentyl propanoate pentanoic acid and propanol pentanol and propanoic acid pentanol and pentanoic acid propanol and propanoic acidarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY