
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![3. In a study of the reaction of compound A and compound B in solution, the following
initial reaction rates were measured at 25°C
[X] [Y] Rate
(mol L-¹)
1.0 x 10-
3.0 x 10**
3.0 x 10-
a. What is the rate law for this reaction?
(mol L-¹)
1.0 x 10-
1.0 x 10-
2.0 x 10-
b. What is the overall order for this reaction?
c. What is the rate constant (k) for this reaction?
(mol L¹ s¹)
3.0 x 10-*
9.0 x 10⁰
3.6 x 10
d. If the initial [X]=1.5 x 10* M and [Y]=5.0 x 10°* M, what is the reaction rate?](https://content.bartleby.com/qna-images/question/9c4f08ab-51ba-41cb-bd75-0ea4532a24f0/5d0f7feb-cacf-4f32-935d-85b07078917d/z8xj102_thumbnail.png)
Transcribed Image Text:3. In a study of the reaction of compound A and compound B in solution, the following
initial reaction rates were measured at 25°C
[X] [Y] Rate
(mol L-¹)
1.0 x 10-
3.0 x 10**
3.0 x 10-
a. What is the rate law for this reaction?
(mol L-¹)
1.0 x 10-
1.0 x 10-
2.0 x 10-
b. What is the overall order for this reaction?
c. What is the rate constant (k) for this reaction?
(mol L¹ s¹)
3.0 x 10-*
9.0 x 10⁰
3.6 x 10
d. If the initial [X]=1.5 x 10* M and [Y]=5.0 x 10°* M, what is the reaction rate?
Expert Solution
arrow_forward
Step 1: Define rate law
 l
l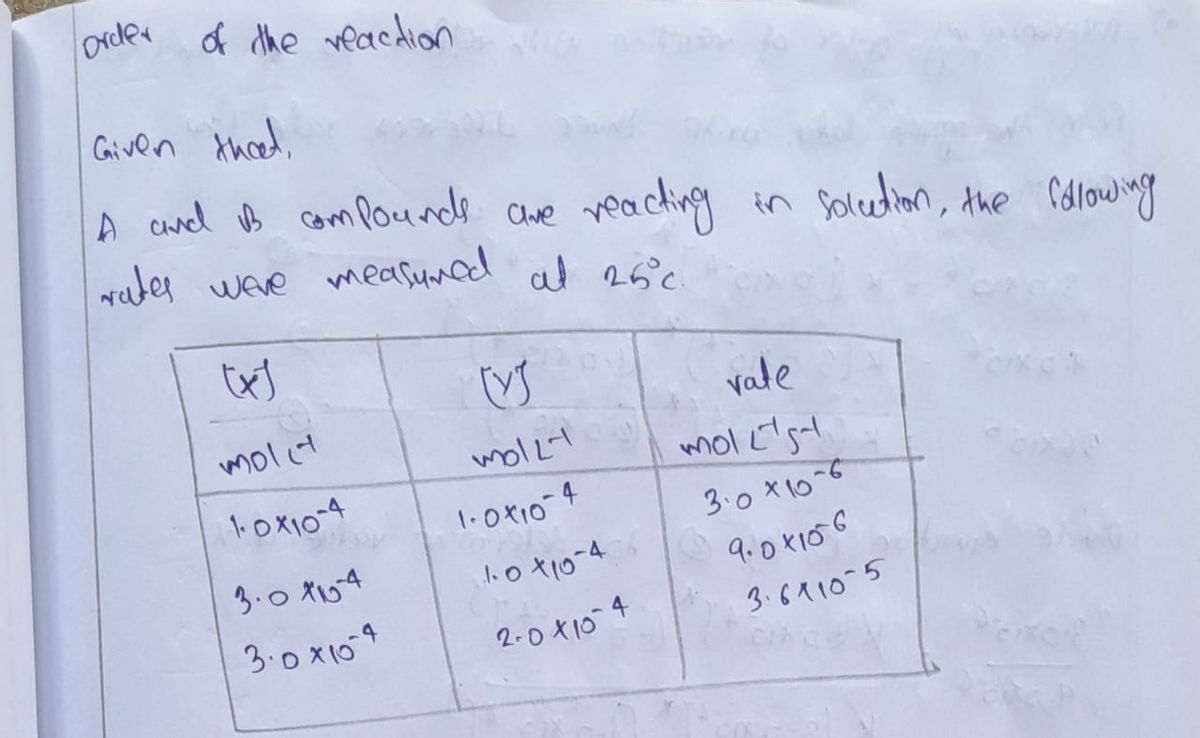
Step by stepSolved in 4 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- calculate the concentration for a reaction A—-> product after 7.0 minutes knowing that the initial concentration is 2.4 M and the rate constant is 3.2×10-3 M-1 sec-1arrow_forwardHow would each of the following change the rate of the reaction shown here? Drag the appropriate items to their respective bins. decreasing the temperature adding some NO(g) Reaction rate increases 2NO(g) + 2H₂(g) → N2(g) + 2H₂O(g) Submit Request Answer Reaction rate decreases removing some H₂(g) P Pearson adding a catalyst Reset Help Reaction rate remains the same.arrow_forwardtab lock atrol D esc Question 14 Based on the initial-rate data below, which is the magnitude (units omitted) for the specific-rate constant for this reaction? Rate (M/s) 1.3 x 10-7 5.2 x 10-7 1.0 x 10-6 [HgCl2] (M) [C2042-] (M) 0.10 0.10 0.10 0.20 0.20 0.20 O 5.2 x 10-6 O 1.3 x 104 O 1.3 x 10-5 O 2.0 x 10-7 Question 15 ! An chemical reaction has specific-rate constant 1.10 x 104 s¹ at 470 C. How many hours are required for a 0.335 M solution to decrea to 0.051 M? 1 O A B Ā N @ 2 W S X 2 option command w # E D DS 4 C R TI % 5 V 2 T G > A 6 MacBook Pro CO B Y 4) & 7 H U N 00 8 J - M ( 9 K O ) O < H 5 L P commandarrow_forward
- The reaction of iodide ion with hypochlorite ion, OCI" (the active ingredient in a "chlorine bleach" such as Clorox), is described in the following equation ocr + I - Or + CI" It is a rapid reaction that gives the following rate data: Initial Concentrations (mol L-1) Rate of Formation of C [OCI-] (mol L-'s-) 1.6 x 10-3 7.6 x 10-3 1.6 x 10-3 1.6 x 10-3 1.75 x 104 8.31 x 104 1.6 x 10-3 9.6 x 10-3 1.05 x 105 What is the rate law for the reaction? rate = rate = rate = rate = k[OC1 ][I]arrow_forward4. Given: A +B O C Rate ( M sec ') 4.0X 10 -5 4.0 X 105 16 X 10-5 [] 0.1 [B] 0.1 0.1 0.2 0.2 0.1 Determine the rate law. Calculate the rate constant. Determine the rate when [A] = 0.05 and [B] = 0.10arrow_forwardAnswer questions 6,7 and 8 please Answer ASAP PLEASEarrow_forward
- 1. Which expression does NOT represent the rate of the following reaction? Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g) b Δ[Mg] ΔΙ b. Δ[Η,] ΔΙ C. -1 2 ΔHCI] ΔΕ d A[MgCl₂] Atarrow_forwardUse the following data table to answer the following questions. Experiment Concn. of A (M) Concn. of B (M) Initial rate (M/s) 1 0.10 0.010 1.2e-3 2 0.10 0.040 4.8e-3 3 0.20 0.010 2.4e-3 d. The rate constant of the reaction is e. The rate of reaction when concentration of A and B are 0.15M and 0.075M isarrow_forward(39 of 41) According to the reaction mechanism in Question 37, please identify all reaction intermediates. A H₂O I H 1) CH3MgBr 2) H3O+ CH3 B OH D F OH X 5 CH3 E H L -H Garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY