
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
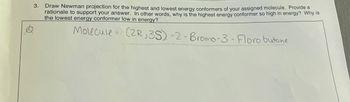
Transcribed Image Text:3. Draw Newman projection for the highest and lowest energy conformers of your assigned molecule. Provide a
rationale to support your answer. In other words, why is the highest energy conformer so high in energy? Why is
the lowest energy conformer low in energy?
Molecule (2R,3S)-2-Bromo-3- Floro butane
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Following is a chair conformation of cyclohexane with the carbon atoms numbered 1 through 6. (a) Draw hydrogen atoms that are above the plane of the ring on carbons 1 and 2 and below the plane of the ring on carbon 4. (b) Which of these hydrogens are equatorial? Which are axial? (c) Draw the alternative chair conformation. Which hydrogens are equatorial? Which are axial? Which are above the plane of the ring? Which are below it?arrow_forwardUse your answers from Problem 2.48 to complete the table showing correlations between cis,trans and axial,equatorial for disubstituted derivatives of cyclohexane. Draw the alternative chair conformations for the cis and trans isomers of 1,2-dimethylcyclohexane, 1,3-dimethylcyclohexane, 1,4-dimethylcyclohexane. (a) Indicate by a label whether each methyl group is axial or equatorial.arrow_forwardConsider the Newman projection below. a. Draw a full Lewis structure of this molecule with R1=Me,R2=Et , and R3=iPr . b. Given the sizes of these R groups (R3R2R1) , does the Newman projection above show thelowest potential energy conformation of this bond? If not, draw a Newman projectionshowing the lowest P.E. conformation (sighting down this same bond). c. To draw a Newman projection in the lowest P.E. conformation, the following rule of thumbusually applies: Place the largest group on the front carbon anti to the largest group on theback carbon. Is your answer to the previous question consistent with this rule of thumb?arrow_forward
- Using your model of butane (CH3CH2CH2CH3) , complete the following graph of the anglebetween the two Me groups vs. potential energy. a. Label each Newman projection of butane on the graph with the words staggered, eclipsed, gauche, and anti, as appropriate. (Note that some structures will have more than one label.) b. Draw a wedge and dash bond representation of butane in its lowest P.E. conformation.arrow_forwardFill in the blanks: cis-1,3-Dimethylcyclohexane has two different chair conformations: one withboth methyl groups in __________ positions and one with both methyl groups in ____________ positions.arrow_forwardPlease show reaekson and don't use hend raitingarrow_forward
- Pertanyaan yang ada pada gambararrow_forward2. Convert the following bond-line structure to Newman projections that represents it lowest energy and highest energy conformations. Look down the indicated C2-C3 bond. lowest energy highest energyarrow_forwardfor each molecule there should be 3 different newman projectionsarrow_forward
- 3. Draw both chair conformations for the cyclohexane molecule shown below, and circle the most stable conformation (the methyl group is larger than the hydroxyl group). OH H3Carrow_forwardWhich of the following is the highest energy chair conformer of the most stable isomer of 1-ethyl-2,4-dimethylcyclohexane? A | B II C) III IV = || ||| > INarrow_forwardCompare the following conformers of butan-2-ol. Match each conformer to their corresponding relative energy. Recall, the more stable the conformer the lower the energy. 1. ОН CH3 CH3 H 2nd Highest Energy 2. HỌH CH3 2nd Lowest Energy H3C OH Highest Energy 3. H3C. CH3 Lowest Energy OH H. CH3 4. >arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning