
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:15
eBook
Print
References
3 attempts left Check my work
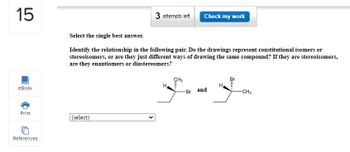
Select the single best answer.
Identify the relationship in the following pair. Do the drawings represent constitutional isomers or
stereoisomers, or are they just different ways of drawing the same compound? If they are stereoisomers,
are they enantiomers or diastereomers?
(select)
H
CH3
-Br
and
H
-CH3
Expert Solution
arrow_forward
Step 1
In the given question, two compounds are given and we have to find the relationship between these two compounds.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Could some one answer these Question 1 and 2 Parts A-B-C I do not need a super detailed explanation i just need to see if did them correctly this is a practice set and is non gradedarrow_forward3 Which is the stereochemical relationship between this pair of molecules?arrow_forwardplease solvearrow_forward
- For the following molecules, draw all possible stereoisomers using line-angle formulas with wedge/dash notation. Label the stereocenters as R or S. Indicate which ones are enantiomers and which ones are diastereomers. (Remember: there are 2 stereoisomers possible for a molecule with n chiral centers) (A) (B) (C) (1 chiral center) Br OH (2 chiral centers) CI OH CH3 (3 chiral centers)arrow_forwardClassify the following pair of compounds as the same compound, enantiomers, diastereomers, constitutional isomers, or not isomeric. Also, select the correct IUPAC name, including the correct (R) or (S) designation, for each. H ÷ ||I The correct IUPAC names are: || J k CI same compound enantiomers diastereomers constitutional isomers not isomeric Compound I: (2R, 3R)-2,3-dichloropentane, Compound II: (2R, 3S)-2,3-dichloropentane Compound I: (2S, 3S)-2,3-dichloropentane, Compound II: (2S, 3S)-2,3-dichloropentane Compound I: (2S, 3R)-2,3-dichloropentane, Compound II: (2S, 3R)-2,3-dichloropentane Compound I: (2R, 3R)-2,3-dichloropentane, Compound II: (2R, 3R)-2,3-dichloropentanearrow_forwardIndicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. Note that cis, trans isomers are an example of stereoisomers. НО. Br F Submit Answer OH F Br Br Retry Entire Group Br 1 more group attempt remainingarrow_forward
- 18 eBook Print References Select the single best answer. Identify the relationship in the following pair. Do the drawings represent constitutional isomers or stereoisomers, or are they just different ways of drawing the same compound? If they are stereoisomers, are they enantiomers or diastereomers? (select) (select) 3 attempts left Check my work H3C 4-4 and CH3 stereoisomers-- diastereomers same compound stereoisomers- enantiomers constitutional isomersarrow_forwarderences) Draw a structural formula of the SR configuration of the compound shown below. CH3 S NR HO Use the wedge/hash bond tools to indicate stereochemistry where it exists. Include H atoms at chiral centers only If a group is achiral, do not use wedged or hashed bonds on it. oodarrow_forwardDoes the molecule below exist as a pair of enantiomers? If so, change the bonds to wedges and dashes to reflect R stereochemistry. If the molecule does not exist as a pair of enantiomers, check the box below. Does not exist as enantiomers Cl CH3 OR Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY