
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
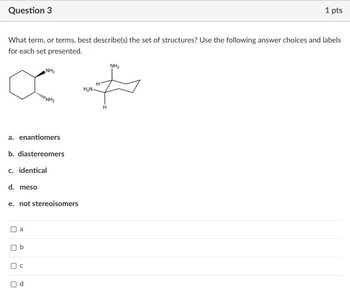
Transcribed Image Text:Question 3
1 pts
What term, or terms, best describe(s) the set of structures? Use the following answer choices and labels
for each set presented.
о
NH₂
NH2
H
H₂N
+
a. enantiomers
b. diastereomers
c. identical
d. meso
e. not stereoisomers
a
b
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- I and II are: I FL O constitutional isomers. O enantiomers. ○ identical. O diastereomers. O not isomeric. F IIarrow_forwardWhat is the relationship between the following pairs of structures? H₂C CH3 H H H H and H H3C CH3 CH3 CH3 H₂C OH H3CH CH₂CH3 CH3 and HO CH₂CH3 H II and III I A. (1) The same molecule, (II) The same molecule, (III) Diastereoisomers B. (1) Diastereoisomers, (II) Enantiomers, (III) The same molecule C. (1) Constitutional isomers, (II) The same molecule, (III) Enantiomers D. (1) The same molecule, (II) Enantiomers (III) Diastereoisomers E. (1) Constitutional isomers, (II) The same molecule, (III) Constitutional isomersarrow_forwardQUESTION 7 Assign R or S configuration to each indicated chirality center (carbon A and B) in the following molecule (Erythronolide B). Which of the following assignments is correct? CH, . CH, B HO, "OH OH CH3 Erythronolide B A a. carbon A (R); carbon B (S) b. carbon A (R); carbon B (R) c. carbon A (S): carbon B (R) d. carbon A (S): carbon B (S)arrow_forward
- Question 2 2 pts Determine the stereochemical configuration on the positions marked (i) and (ii) in the structure below. A OH CO₂H Br A. (i) S and (ii) E B. (i) Rand (ii) Z C. (i) R and (ii) E D. (i) S and (ii) Z A B ОЕarrow_forwardQuestion 7 of 23 > Identify the chirality center(s) (sometimes called chiral atom) in the compound shown. Identify the chirality center(s). ОН ОН ОН О Н ОН С-5 H-C- -C- C-H С-1 C-6 H H H H H С-4 C-3 How many stereoisomers exist for this compound? С-2 stereoisomers:arrow_forwardPlease don't provide handwritten solutionarrow_forward
- 3 Which is the stereochemical relationship between this pair of molecules?arrow_forwardC/ What is the relationship between these two structures? CI same enantiomers constitutional isomers diastereomersarrow_forwardNH2 Br OH HS CI N. N. Draw the stereoisomer where all the chiral centers have the R configuration. ZI IZarrow_forward
- Identify the relationship between these two structures:arrow_forward27. The relationship between the two molecules drawn below is: CH3 -Br H. Br2HC H' CH3 Br a. enantiomers b. diastereomers c. identical / conformational isomers / meso compound d. constitutional isomers e. different compounds (not isomers)arrow_forwardQuestion 6 What is the relationship between the two structures shown? CH3 -H Br H H Br CH3 enantiomers O diastereomers O these are the same compound O constitutional isomers H I Br CH3 H Br CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY