
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help with row 3:
Follow the instructions in each column. Hint for the last column: draw it with a wedge and again with a
dash – which gives the correct configuration?
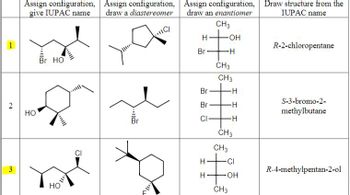
Transcribed Image Text:**Transcription for Educational Purposes**
This educational content focuses on stereochemistry and the assignment of configurations for different organic compounds. It includes examples of diastereomers, enantiomers, and IUPAC naming. Below is a detailed explanation of each item:
### Row 1
1. **Assign configuration, give IUPAC name:**
- Structure: A molecule with a chiral center, including a bromine (Br) and a hydroxyl group (OH).
- IUPAC Name: The corresponding name is **R-2-chloropentane**.
2. **Assign configuration, draw a diastereomer:**
- Diagram: A cyclic compound with a different spatial arrangement involving chlorine (Cl).
3. **Assign configuration, draw an enantiomer:**
- Fischer Projection: The structure is presented vertically with respective groups Br, OH, H, and CH3 around the chiral center.
4. **Draw structure from the IUPAC name:**
- IUPAC Name provided: **R-2-chloropentane**.
### Row 2
1. **Assign configuration, give IUPAC name:**
- Structure: Contains a cyclic structure with a chiral center, featuring Br and OH groups.
- IUPAC Name: **S-3-bromo-2-methylbutane**.
2. **Assign configuration, draw a diastereomer:**
- Structure: Linear compound with Br substituents.
3. **Assign configuration, draw an enantiomer:**
- Fischer Projection: Arrangement of CH3, Br, H, Cl along a central axis.
4. **Draw structure from the IUPAC name:**
- IUPAC Name provided: **S-3-bromo-2-methylbutane**.
### Row 3
1. **Assign configuration, give IUPAC name:**
- Structure: Contains a cyclic structure with multiple chiral centers, featuring Cl and OH groups.
- IUPAC Name: **R-4-methylpentan-2-ol**.
2. **Assign configuration, draw a diastereomer:**
- Structure: Cyclic compound featuring different spatial configuration involving Fluorine (F).
3. **Assign configuration, draw an enantiomer:**
- Fischer Projection: Features groups CH3, H, Cl, OH around a central axis
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 8 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compounds whose structures are shown below would have ÓH ÓH ÓH OH Select one: the same melting point. O different melting points. equal but opposite optical rotationsarrow_forwardDraw a constitutional isomer of the molecule below. Try to draw it so that it does not have any rings.arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table.arrow_forward
- To preview image Click here With respect to substituent Me, determine whether substituent Br is cis or trans. 1. Choose the correct option for A Cis 2. Choose the correct option for B Trans 3. Choose the correct option for C Cis C/₁ A ***||| Et Et B CI Et C CIarrow_forwardPart II (70 points) Directions: Answer each of the questions that follow. Please read the directions for each question carefully. When structures are required please draw them Clearly and Neatly. Make sure questions are numbered clearly as well. 1. (12 points; 2 points each) Give a correct name for each of the following compounds: CH3 A) CH—CH,C—CHICHCHCH, CH OH alf B) CH-CH₂- CH₂ -CH₂-CH₂arrow_forwardConsider the mechanism of the acid-catalyzed cleavage of the ether shown below. excess HBr heat Br Br O Complete the mechanism by adding curved arrows and products. Add steps as necessary and be sure to add lone pairs and charges where relevant. 田 C to :0 + Add/Remove step Xarrow_forward
- Solve both please neat and clean...arrow_forwardITEM #5 (Everyone should look at this question). Consider the two Fischer projections shown at right. They CH CH are NOT of the same molecule. H- -HO- HO In a short paragraph, explain why the molecules shown are NOT the same. OH но- H. HO. но In your explanation, describe how the groups of a Fischer projection are positioned in the ACTUAL molecule (Are the molecules flat in real life, or three-dimensional in real life? If three-dimensional, where exactly are the Hs and OHs positioned when one interprets these projections?) H- HO но H- CH,OH ČH;OH Also in your explanation, describe why the second molecule is not simply the first molecule flipped over (like a pancake).arrow_forwardCheck the box under each compound that exists as a pair of mirror-image twins. If none of them do, check the none of the above box under the table. OH O || HỌ—CH2—CH—CH HỌ—CH2 -CH₂-OH none of the above OH H-C- CH-C-H C1O CH3 O CH3-CH-C-CH₂-OH X 5arrow_forward
- Inspect the structures shown below and select the correct statement. COƏH HN-C-H CH2 ÇOH H2N-C-H CH2 COH HN-C-H CH2 COH HN-C-H Он SH 1 2 4 O #1 and #4 have the S configuration, #2 and #3 have the R configuration. O All structures have the R configuration. O All structures have the S configuration. O #1, #2, and #4 have the S configuration and #3 has the R configuration. O #1 and #4 have the R configuration, #2 and #3 have the S configuration.arrow_forwardcheck all that applyarrow_forwardCurved Arrows-start at a pair of electrons. Not an atom or a charge. Assume any stereocenters are (S). SN2 Curved Arrows (H3C)2CHCH₂CH₂CH₂C(Br)CH3 SN1 Curved Arrows (H3C)2CHCH₂CH₂CH₂C(Br)CH3. NaOCH₂CH3 HOCH₂CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY