Question
thumb_up100%
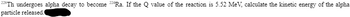
Transcribed Image Text:228Th undergoes alpha decay to become 224Ra. If the Q value of the reaction is 5.52 MeV, calculate the kinetic energy of the alpha
particle released.
Expert Solution
arrow_forward
Concept and Principle:
- When an unstable nucleus undergoes alpha decay they release a helium nucleus or alpha particle. This kind of decay is called alpha decay.
- The following reaction takes place in alpha decay.
Here X and X' represent different nuclei.
- The kinetic energy of the alpha particle released is given by,
Here A is the atomic number of the parent nucleus and Q is the energy released in the reaction.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- The half life for the radioactive decay of Ce-141 is 32.5 days. If the sample has an activity of 4.0 úCi after 130 days have elapsed, what was the initial activity, in microcuries, of the sample?arrow_forwardA 162.0-mCi sample of a radioactive isotope is purchased by a medical supply house. If the sample has a half-life of 14.0 days, how long will it keep before its activity is reduced to 24.0 mCi? Answer:_______ daysarrow_forwardNeutron probes are used in agronomy to measure the moisture content of soil. A pellet of 241 Am emits alpha particles that cause a beryllium disk to emit neutrons. These neutrons move out into the soil where they are reflected back into the probe by the hydrogen nuclei in water. The neutron count is thus indicative of the moisture content near the probe. What is the energy of the alpha particle emitted by the 241^Am? Answer in megaelectron voltsarrow_forward
- There is more than one isotope of natural uranium. If a researcher isolates 0.7 mg of the relatively scarce 235U and finds this mass to have an activity of 78.0 Bq, what is its half-life in years? =___yearsarrow_forwardThe isotope of Curium 242Cm has a half-life of 160 days and emits alpha particles with an energy 6.1MeV. What is the power emitted by a sample of 242Cm with a starting number of N0 = 1018 atoms after 80 days?arrow_forward
arrow_back_ios
arrow_forward_ios