Question
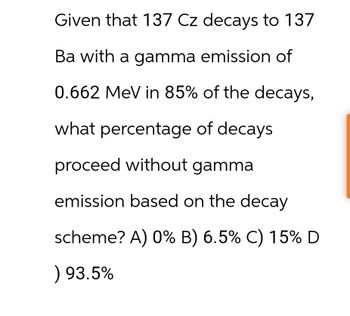
Transcribed Image Text:Given that 137 Cz decays to 137
Ba with a gamma emission of
0.662 MeV in 85% of the decays,
what percentage of decays
proceed without gamma
emission based on the decay
scheme? A) 0% B) 6.5% C) 15% D
) 93.5%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- A certain isotope has a half-life of 5.9 h and an atomic mass of 95.98 u. What will the activity in Bq of a 1.36-g sample be after 19.8 h?arrow_forwardWhat is the half‑life of an isotope that decays to 6.25%6.25% of its original activity in 24.2 h?24.2 h? half‑life:arrow_forwardCalculate the energy released by the fission of 5 gm of 235U in kWh if energy released per fission is 200 MeV. a) 1.13 X 105 kWh b) 2.65 X 105 kWh c) 3.78 X 105 kWh d) 4.12 X 105 kWharrow_forward
- Experimental values are obtained from actual experiments. The model represents a theoretical approximation. I need B/A value of for sodium please calculate it by using the given formula in the discussionarrow_forwardE c) For a radio-nuclide, a number No of atoms at time t = 0 decays as N(t) = No e¯‹, where A is the decay constant. i) Derive the relationship between the half-life t₁ and the time constant □ = = 1/λ. ii) The radio-nuclide radium-226 has a half-life of 1600 years. Calculate the activity of one gram of radium in Becquerels. iii) Radium-226 decays to radon-222, known as the daughter product. The amount of a daughter product present will vary with time, Na(t). Sketch two graphs, with appropri- ate axes and labels, to show schematically how N(t) and Na(t) evolve if Na(0) = 0, for the two extreme cases where the daughter product is much more radioactive than the parent (\d >> \) and where the daughter product is much less radioactive than the parent (\a << λ). Given that the half-life of radon-226 is 3.8 days, indicate which graph is relevant and estimate the amount of radon in equilibrium with one mole of radium.arrow_forwardThe mass defect of nuclei (139X ) is 1.693 amu. Estimate the total binding energy per nucleon in J/nucleon. (c = 2.998 x 108 m/s) Note: Express your answers in three decimal places in scientific notationarrow_forward
- in this nucluar reaction ¹¹B(d,α)⁹Be . if the Deuterium fall down with kinetic energy 2.5 Mev , while alpha's particles start moving with kinetic energy 8.5 Mev out by angle 90 degree , calculate : a- calculate value of Q for this reaction then note is it Exothermic or Endothermic reaction. b- calculate the Critical energy of this reaction .arrow_forwardWhich of the following formula is used to calculate the decay constant λ which is related to the half-life (T)?arrow_forwardOne of the fusion reactions that takes place as a star ages is called the triple alpha process. 3 42He → 126C Calculate the mass defect (in u) and the energy produced (in MeV) each time the reaction takes place.arrow_forward
arrow_back_ios
arrow_forward_ios