
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The problems that are listed below need to be solved and you may access those problems via viewing them through the attached images in this request. To emphasize more, the problems are 2.29; 2.30; 2,31; 2.32; 2.33; 2.43.

Transcribed Image Text:2.29. WP A seed crystal of diameter D (mm) is placed in a solution of dissolved salt, and new crystals are observed to nucleate
(form) at a constant rate r (crystals/min). Experiments with seed crystals of different sizes show that the rate of nucleation varies
with the seed crystal diameter as
r(crystals/min)
200D – 10D²
(Din mm)
a. What are the units of the constants 200 and 10? (Assume the given equation is valid and therefore dimensionally
homogeneous.)
b. Calculate the crystal nucleation rate in crystals/s corresponding to a crystal diameter of o.050 inch.
c. Derive a formula for r(crystals/s) in terms of D(inches). (See Example 2.6-1.) Check the formula using the result of Part (b).
Exploratory Exercise-Research and Discover
d. The given equation is empirical; that is, instead of being developed from first principles, it was obtained simply by fitting an
equation to experimental data. In the experiment, seed crystals of known size were immersed in a well-mixed supersaturated
solution. After a fixed run time, agitation was ceased and the crystals formed during the experiment were allowed to settle to
the bottom of the apparatus, where they could be counted. Explain what it is about the equation that gives away its empirical
nature. (Hint: Consider what the equation predicts as D continues to increase.)
2.30. WP The density of a fluid is given by the empirical equation
p = 70.5 exp(8.27 × 10-7P)
where p is density (lbm/ft³) and P is pressure (lbf/in²).
a. What are the units of 70.5 and 8.27 × 10-7?
b. Calculate the density in g/cm3 for a pressure of 9.00 × 10° N/m².
Answer
c. Derive a formula for p(g/cm³) as a function of P(N/m²). (See Example 2.6-1.) Check your result using the solution of Part (b).
BIOENGINEERING
2.31. WP The volume of a microbial culture is observed to increase according to the formula
V(cm³) = aebt
where t is time in seconds.
a. Calculate the expression for V(in°) in terms of t(h).
b. Since both the exponential function and its argument must be dimensionless, what must be the units of a and b?
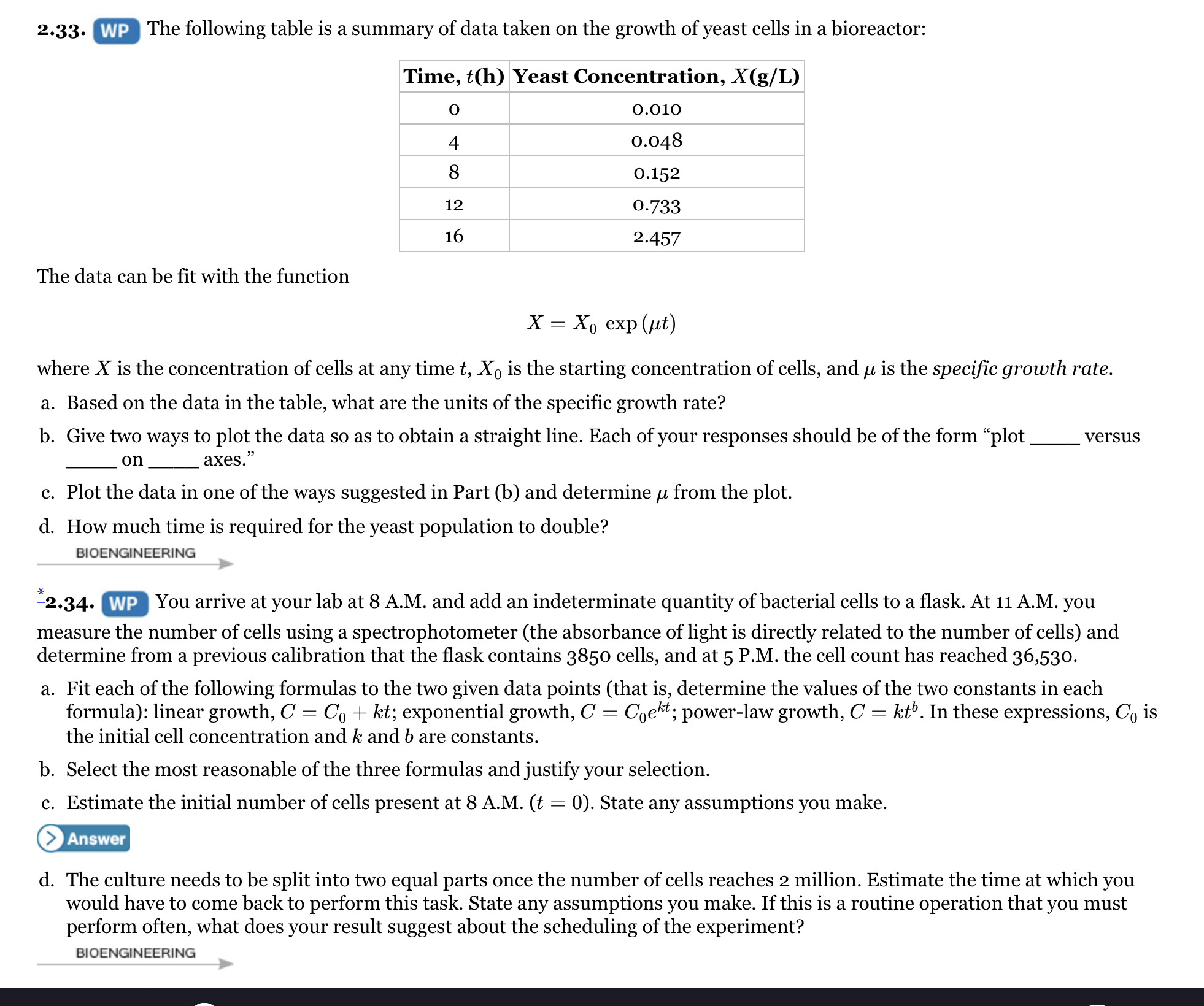
Transcribed Image Text:2.33. WP The following table is a summary of data taken on the growth of yeast cells in a bioreactor:
Time, t(h) Yeast Concentration, X(g/L)
0.010
0.048
8
0.152
12
0.733
16
2.457
The data can be fit with the function
X — Хо ехp (ut)
where X is the concentration of cells at any time t, X, is the starting concentration of cells, and u is the specific growth rate.
a. Based on the data in the table, what are the units of the specific growth rate?
b. Give two ways to plot the data so as to obtain a straight line. Each of your responses should be of the form "plot
versus
on
axes."
c. Plot the data in one of the ways suggested in Part (b) and determine u from the plot.
d. How much time is required for the yeast population to double?
BIOENGINEERING
-2.34. WP You arrive at your lab at 8 A.M. and add an indeterminate quantity of bacterial cells to a flask. At 11 A.M. you
measure the number of cells using a spectrophotometer (the absorbance of light is directly related to the number of cells) and
determine from a previous calibration that the flask contains 3850 cells, and at 5 P.M. the cell count has reached 36,530.
a. Fit each of the following formulas to the two given data points (that is, determine the values of the two constants in each
formula): linear growth, C = C, + kt; exponential growth, C = Coekt; power-law growth, C = kt'. In these expressions, Co is
the initial cell concentration and k and b are constants.
b. Select the most reasonable of the three formulas and justify your selection.
c. Estimate the initial number of cells present at 8 A.M. (t = 0). State any assumptions you make.
Answer
d. The culture needs to be split into two equal parts once the number of cells reaches 2 million. Estimate the time at which you
would have to come back to perform this task. State any assumptions you make. If this is a routine operation that you must
perform often, what does your result suggest about the scheduling of the experiment?
BIOENGINEERING
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- answers given steps how to reach these pleasearrow_forwardGiven a transfer function G (s) = determine the range of K to s4 +0.2s3 –s+K’ make G(s) stable. Empty set Oarrow_forward3-19. Draw the block diagram representing the following transfer functions. In each case, do not do any algebraic manipulations to simplify the transfer functions, but use the rules of block diagram algebra to simplify the diagram if possible. (a) Y(s) = (b) (c) Ctrl Shift Tab Y(s) = Y₁(s) = Y₂ (s) CapsLk [XIDOLBY AUDIO Esc FnLock 3-20. Determine the transfer function C(s)/R(s) for the 5°C Mostly cloudy H Q Search BOUR Fn K₁ TIS + 1 1 2 Q A TS+1 = G₁(s) X (s) + G3 (s) Y2 (s) = G₂ (s) Y₁(s) @ Z 240 F W S Privacy Shutter on Webcam 360" Versatility Front Facing Speakers SO ·X (s) + ideapad Flex 5 4 [K₁ F1(s) - K₂ F2 (s)] 3+ 14+ # 3 X Alt E 220 K₂ T2S + 1 D CH C V X(s) QF+ 6 G Y B & 7 H U N 8 J M 9 F10 K O L Alt P Ctrlarrow_forward1. Indicate whether the following systems described by the transfer function Y'(s) = G(s)U'(s) are stable or unstable. (a) G(s) = 2 (b) G(s) = 2 (c) G(s) = (d) G(s) = Ts+1 (indicate the range of values of t for which the system is stable) (e) G(s) = -s-6 (f) G(s) = s-6 s-3 (g) G(s) = 772+26T8+1 stable) T > 0 (indicate the range of values of s for which the system is (h) G(s) = e -3s (1) G(s) -3s (indicate the range of values of T for which the system is stable) Ts+1arrow_forwardUsing method 1 on the picturearrow_forwardYour Question :Your Question :Your Question :Your Question :help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!arrow_forwardWrite out the steps (including equations) for BUBL P, DEW P, BUBL T, and DEW T calculations using the modified Raoult’s law.arrow_forwardFor the transfer function given by: G(s)=8/((s+2)(2s^2+3s+4)) perform the stability analysis using the Routh-Hurwitz test.arrow_forwardi need help with all parts of this question asaparrow_forwardTYPEWRITTEN ONLY PLEASE UPVOTE. DOWNVOTE FOR HANDWRITTEN. DO NOT ANSWER IF YOU ALREADY ANSWERED THISarrow_forwardThe kinetics of some phase transformations obey the Avrami relationship. Using the fraction transformed- time data given below, determine the total time required for the transformation to go to 94% completion. Fraction transformed Time (s) 0.2 0.7 11.6 26.7 Sarrow_forwardarrow_back_iosarrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The