
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
Question 2
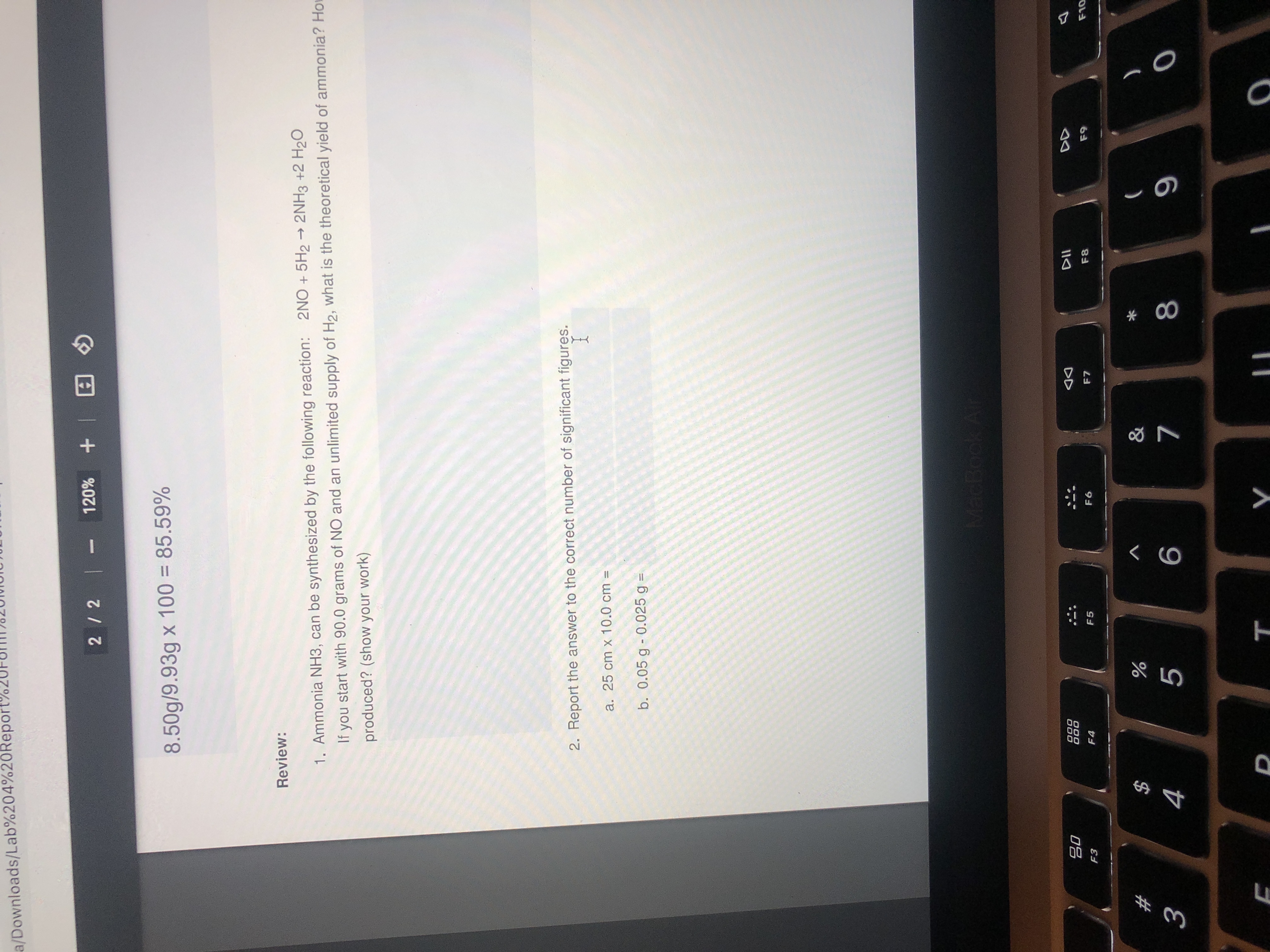
Transcribed Image Text:2. Report the answer to the correct number of significant figures.
a. 25 cm x 10.0 cm =
b. 0.05 g - 0.025 g =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- I. Significant Figures and Conversion A. Scientific Notation 1. 58 cm → km 2. 67 W → MW 3. 196 mL L 4. 17 dm – cm 5. 299 W → TWarrow_forwardDo the following calculations to the correct number of significant figures: MUST follow the rules of SIGNIFICANT figures a.) 432/7.3-28.523 b.) 0.004 + 0.09879 c.)87.6 + 9.888 +2.3 + 100.77 d.) 5.01 x 105/7.8 x 102arrow_forwarda. Using the correct rules for multiplication/division and significant figures provide the answer for the following calculation. 6.98 x 89.44 = b. Using the correct rules for multiplication/division and significant figures provide the answer for the following calculation. 7831 x 76.987 = c. Using the correct rules for addition/subtraction and significant figures provide the answer for the following calculation. 7831 + 76.987 = d. Using the correct rules for addition/subtraction and significant figures provide the answer for the following calculation. 7831.67 + 76.987 = e. Using the correct rules for addition/subtraction and significant figures provide the answer for the following calculation. 7831.67 + 76.987 =arrow_forward
- 2. How many significant figures are in the following? a. 50.0 cm b. 0.00500 m c. 1.50 x 105 secarrow_forwardIII. Perform these calculations and express the result with the proper number of significant figures. a. (4.850 g – 2.34 g)/1.3 mL b. (4.66 x 10-3) × 4.666 c. 0.003400/65.2arrow_forward2. Evaluate each of the following expressions. State the answer to the proper number of significant figures. a) 73.74 kg - 27.23 kg = b) 500 feet - 35 feet = = d) 0.050 km x 15.38 km x 780.2 km - c) 64.0 g = 16.0 mLarrow_forward
- 2. An object has a volume of 0.0012 m3 and a mass of of 2.26 kg. Calculate the density of the object in g/cm3.arrow_forward3. A pure 3.88 kg sample of a solid occupies a volume of 66.0 mL. Calculate its density in g/mL to the correct number of significant figures. Density = mass/volume Group of answer choices a) 58.8 g/mL b) 5.88 x 10^-5 g/mL c) 0.059 g/mL d) 0.0588 g/mLarrow_forwardcalculate the mass of water in metric tons. in a small lake with an average depth of 5.3 m and a surface area of 4.0 hectares. A metric ton = 1000 kg. a hectare is the area covered by a square measuring 100m by 100marrow_forward
- You measure the distance between to cities to be 42.0 miles. You need to send this data to Brazil which uses metric. Convert 42.0 miles to km. 5285 ft = 1 mile 12 in = 1 ft 2.54 cm = 1 in Use the conversion factors provided and express using correct significant figures and no unit.arrow_forward1. Suppose some measurements are made on two different homogeneous stones to find out if they are made of the same kind of rock. The mass and volume measurements are listed below. Are the two stones the same type of rock? Why or why not? Show all calculations. Mass Volume 58.0 grams 50.1 grams 20.0 cm3 15.0 cm3 Stone 1 Stone 2arrow_forward3. Provide the answer with the appropriate number of significant figures. a. 6.03 x 650 b. 2.7 x 1031.645 c. 3.000 + 7.61 + 124.2 d. (3.55 x 109) x (9.5 x 10-7) e. 7.845 - 1.20 f. 7.845- 1.2 g. (6.72 x 106 ) - (4.32 x 10“ )arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning