
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
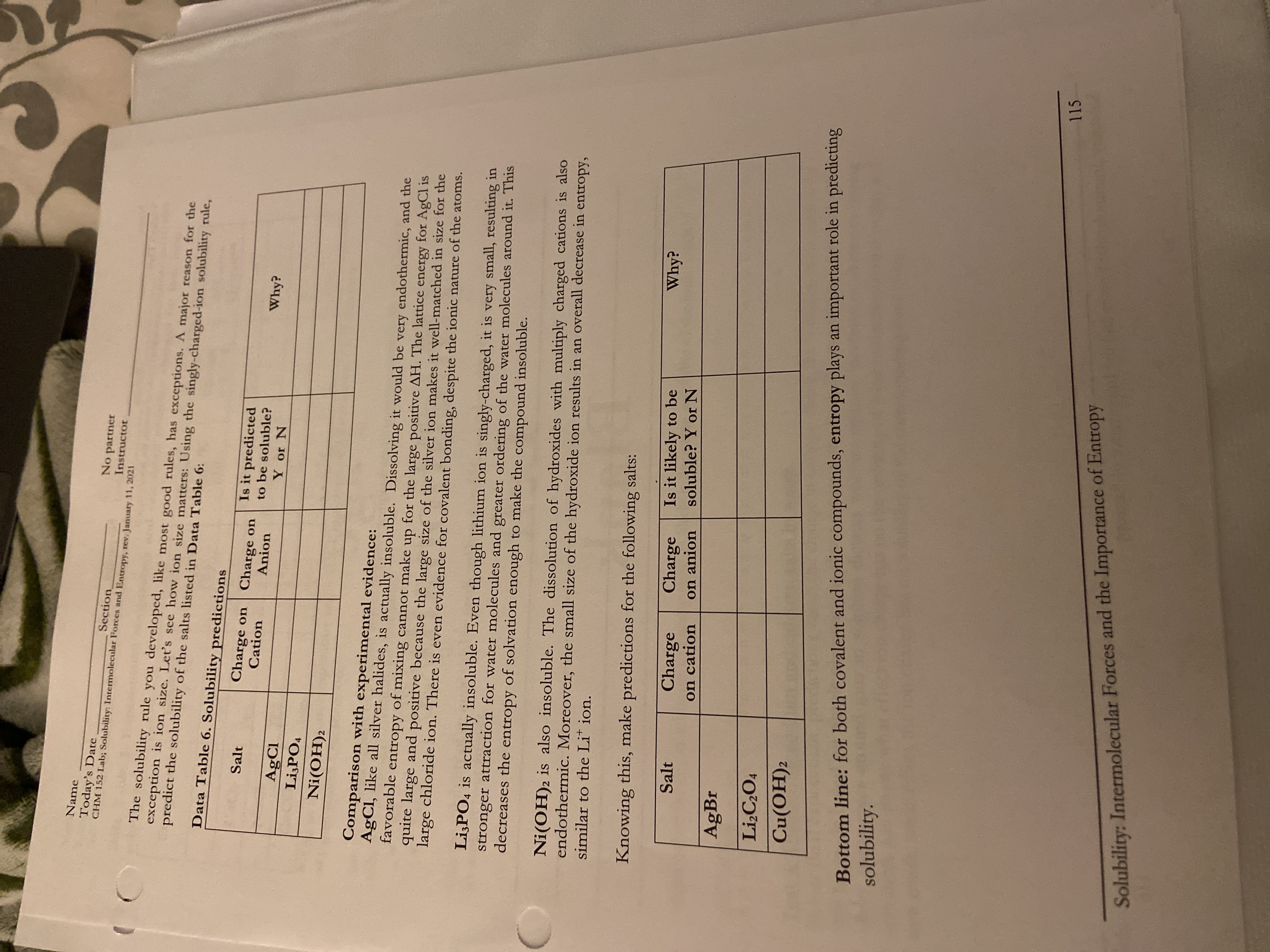
I need help with the blank tables on the page as well as the question on the bottom
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need help on this it’s hard and I keep trying but it still don’t work.?arrow_forwardConsider heating solid water (ice) until it becomes liquid and then gas (steam) (Figure 1). Alternatively, consider the reverse process, cooling steam until it becomes water and, finally, ice ( Figure 2). In each case, two types of transitions occur, those involving a temperature change with no change in phase (shown by the diagonal line segments on the graphs) and those at constant temperature with a change in phase (shown by horizontal line segments on the graphs). Figure Temperature (°C) 100 0 Heating curve Liquid and gas in equilibrium Solid and liquid, in equilibrium Solid Liquid Heat added (J) 1 of 2 associated with a change in phase at constant temperature is given by q=mΔΗ where q is heat in joules, m is mass in grams, and AH is the enthalpy in joules per gram. Physical constants The constants for H₂O are shown here: ▼ . Specific heat of ice: Sice = 2.09 J/(g. °C) Specific heat of liquid water: Swater = 4.18 J/(g. °C) Enthalpy of fusion (H₂O(s)→H₂O(1)): AHfus = 334 J/g Enthalpy…arrow_forwardPart A Be ion Express your answer as a signed integer in the form 3+. Request Answer Previous Answers Submit Incorrect; Try Again; 3 attempts remaining Part B Sc ion Express your answer as a signed integer in the form 3+. MacBook Airarrow_forward
- can you type the answe because i cant read your hand writingarrow_forwardWhat happens when the kinetic energy of particles in a liquid state increases? A The particles vibrate more slowly, causing the forces of attraction holding them together to increase, resulting in a solid. C D The particles vibrate fast enough to overcome the forces of attraction holding them together and become a gas. The particles vibrate so slowly that the particles are no longer held together, resulting in a gas. The particles vibrate so quickly that the particles ionize and become plasma.arrow_forwardAre my answers right?arrow_forward
- Need help with questions 1 and 3. Please see attached image. For question 3, tell me where I need to draw the arrows from and to.arrow_forwardHow do I apply this in the answer chart that was given? in other words how do I write out the entire question.arrow_forwardI don't know how you got the answer, can you plz do the math step by step.arrow_forward
- It is sometimes necessary to take powers or roots to solve chemistry problems. Take powers and roots as needed on your calculator to complete the following: 2.234 a = 6³ = 14.9 = C = 2.004.30 d².48 = 10.6 a = b= = C = d =arrow_forwardHow do you do number 2 and 3?arrow_forwardI have a hard time with this questionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY