
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please solve number 2 and explain
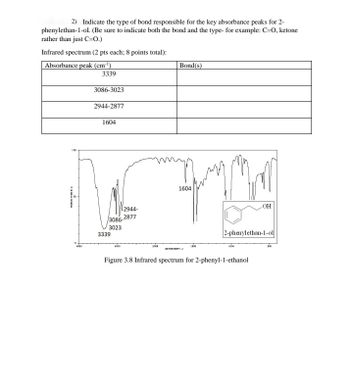
Transcribed Image Text:2). Indicate the type of bond responsible for the key absorbance peaks for 2-
phenylethan-1-ol. (Be sure to indicate both the bond and the type- for example: C-O, ketone
rather than just C=0.)
Infrared spectrum (2 pts each; 8 points total):
Absorbance peak (cm³¹)
3339
100
4000
3086-3023
2944-2877
1604
3086-
3023
3339
2944-
2877
1000
Bond(s)
1604
муз
2-phenylethan-1-ol
1000
OH
Figure 3.8 Infrared spectrum for 2-phenyl-1-ethanol
$30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What do the results of these three experiments demonstrate when compared to the true density of water?arrow_forward2. Why are similar sized organic molecules that contain oxygen atoms more soluble in water than organic molecules that only contain carbon and hydrogen atoms? Give examples to support your explanationarrow_forwardRank the boiling points of the molecules shown below from lowest to highest. 7th attempt Jhi See Periodic To! Question List (4 images) (Drag and drop into the appropriate area) No more items Correct Answer List SUBMI VIEW SOLUTION 22 OF 25 QUESTIONS COMIPLETED MacBook Airarrow_forward
- Use the particle model to explain how the size of the particles would affect the rate of diffusion.arrow_forwardE DetaMath 6 Quiz Chemistry Spring Final x D A sample of helium gas has xG 391ml of gas with a tempera x G number of electrons on a tric + -> b cfbisd.instructure.com/courses/85698/quizzes/390093/take/questions/2865049 Q * e D GO D CFB A Pearson Realize 6 Dashboard @ Student Resources E Google Docs creat A google ciassroom O Nearpod Welcome- O Meeting is in progre O Reading list Balance the following reaction: Al + Fe203 → Al203 + Fe *remember to enter a 1 for coefficient is there is no change. US VO 3:53 esc @ #3 $ bac 3 4 7 8. 9. W e t y a d. f karrow_forwardDetermine the net force necessary to give an object with a mass of 2.0 k an acceleration of 5.8 m/sarrow_forward
- When the temperature is lowered, the speed at which molecules move is slowed. O True False y cloudy Search aarrow_forwardPart B What are each of the following observations an example of? Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help When person applies perfume in one corner of the room you can smell its fragrance in another room. When a coworker microwaves popcorn, you can smell the vapors in your office 5 minutes later. A balloon in a constant environment gets smaller and smaller over the course of a week as gas leaks through small holes in the rubber. When you drive over a nail the volume of the tire decreases slowly over time as air escapes through the hole. Diffusion Effusionarrow_forward7:10 Which microscopic representation best represents a diatomic gas? answers..arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY