
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:FUL2 9
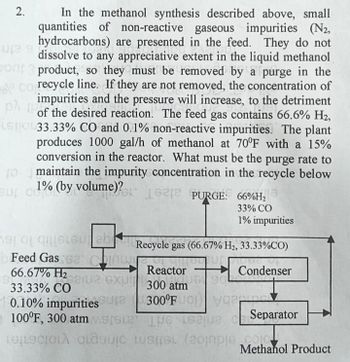
In the methanol synthesis described above, small
quantities of non-reactive gaseous impurities (N2,
hydrocarbons) are presented in the feed. They do not
dissolve to any appreciative extent in the liquid methanol
on product, so they must be removed by a purge in the
corecycle line. If they are not removed, the concentration of
impurities and the pressure will increase, to the detriment
of the desired reaction. The feed gas contains 66.6% H₂,
ELSTOL 33.33% CO and 0.1% non-reactive impurities. The plant
produces 1000 gal/h of methanol at 70°F with a 15%
conversion in the reactor. What must be the purge rate to
(0 maintain the impurity concentration in the recycle below
uc1% (by volume)? Gre PURGE: 66%H₂
118
33% CO
1% impurities
2.
mas o que eu ab
Feed Gas 62' Chin
66.67% H₂
33.33% CO
0.10% impuritiessure
Separator
100°F, 300 atm MS
e eue C
relaciona clasic spel (20mple Methanol Product
122102 exu
Recycle gas (66.67% H₂, 33.33%CO)
Condenser
Reactor
300 atm
300°FUO Viney
QUGI SOL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- Carbon monoxide combines with chlorine in the presence of a suitable catalyst to form phosgeneaccording to the following reaction. CO (g) + Cl 2 (g) COCl 2 (g). After reaction, the products contained 12 moles of phosgene, 3 moles of chlorine and 8 moles ofcarbon monoxide. Calculate the following: (i) Limiting reactant and excess reactant (ii) The percentexcess reactant used (iii) Degree of completion and conversion (iv) Extent of reaction for COCl 2 and(v) Yieldarrow_forwardChoose four ways in which the yield of ammonia in the reaction below can be improved for a given amount of H2. N2 (g) + 3H2 (g) ---- 2NH3 (g), deltaH<0 (Select all that apply.) decreasing the nitrogen or hydrogen concentration increasing the temperature increasing the total pressure on the mixture removing the gaseous or from the equilibrium by liquefying removing the gaseous from the equilibrium by liquefying it decreasing the total pressure on the mixture lowering the temperature increasing the nitrogen concentrationarrow_forwardTo no one's surprise, the only one who's been getting burned is Wile E. Coyote. Good thing he has a few ACME brand instant cold packs! These cold packs contain ammonium nitrate and water, separated by a thin plastic divider. When the divider is broken, the ammonium nitrate dissolves according to the following endothermic reaction: NH,NO3(s) → NH,+(ag) + NO3 (ag) As Wile E. Coyote ices his wounds and plans his next move, he wants to measure the enthalpy change for this reaction (maybe he's smarter than he seems!). 1.05 g of NH,N03 is dissolved in enough water to make a solution with a mass of 27.0 g. The initial temperature is 26.3 °C and the final temperature (after the solid dissolves) is 20.7 ° C. Part A Based on the information given, what is the total heat (gryn) associated with the dissolution of the NH,NO3 ? You may assume that the solution that forms has a heat capacity equivalent to water, 4.184 J. g 1.º C-1 Express your answer to two significant figures. qrxn = Jarrow_forward
- Alumina from bauxite separation stage calculations A crucial step in the production of aluminum from bauxite ore is the separation of alumina from the remaining mineral impurities in the ore. In the Bayer process this is accomplished by treating bauxite with aqueous NaOH to produce NaAIO2 (alumina). NaOH(aq) + Al(OH)3(s) → NaAIO2(aq) + 2H2O(1) Since NaAIO2 is water soluble while the residual mineral constituents of bauxite are not, a separation can be achieved by allowing the minerals to settle out and decanting the aqueous solution of NAAIO2 and unreacted NaOH. In order to further recover any NAAIO2 entrained in the settled mineral solids, this "mud" is repeatedly washed with water and allowed to settle, and the wash water is decanted. The figure below shows one stage of this washing-settling process. Wash water Slurry - Open to air Mixer/Wash tank Setling tank Decanted solution System boundary Washed mud In this stage, a feed slurry consisting of 10 wt% solids, 11 wt% NaOH, 16 wt%…arrow_forward3) A common method used in manufacturing sodium hypochlorite bleach is by the reaction a, + NaOH → NaCI + NaOCI + H,0 Chlorine gas is bubbled through an aqueous solution of sodium hydroxide, after which the desired product is separated from the sodium chloride (a by-product of the reaction). A water-NaOH solution that contains 1145 Ib of pure NaOH is reacted with 851 Ib of gaseous chlorine. The NaOCl formed weighs 618 Ib. a) What was the limiting reactant? b) What was the percentage excess of the excess reactant used? c) What is the degree of completion of the reaction, expressed as the moles of NaOCI formed to the moles of NaOCI that would have formed if the reaction had gone to completion? d) What is the % yield of NaOCI per amount of chlorine used (on a weight basis)?arrow_forward#3 The first step in the reaction sequence for the production of nitric acid via the oxidation of ammonia is: 4NH3 + 502 2 4NO + 6H2O 75% conversion is achieved with an equimolar mixture of ammonia and oxygen fed at the rate of 100 mol/h. Determine the outlet compositions. (Hint: determine the limiting reactant).arrow_forward
- For the preparation of methyl iodide, 2000lb/day of hydroiodic acid are added to an excess methanol (HI + CH3OH CH31 + H2O). If the product contains 81.6% along with the unreacted methanol and the waste contains 82.65% HI and the 17.35% H2O, calculate, assuming that the reaction is 40%complete in the vessel: a. The weight of the methanol added per day b. The amount of HI recycled Recycled HI (R) 2000 lb/day HI- SEPARATOR Waste (W) 82.65% HI 17.35% H₂O 40% complete REACTOR CH₂OH(X) Product (Y) 81.6% CH₂l 18.4% CH3OHarrow_forwardThe successive reactions in the manufacture of HCI from salt and sulfuric acid may be represented by the following equations: NaCl + H2SO4 = NaHSO4 + HCINaCI + NaHSO4 = Na2SO4 + HCl In practice the salt is treated with aqueous sulfuric acid containing 75% H2SO4 in slight excess of the quantity required to combine with all the salt to form Na2SO4. Although the first reaction proceeds readily, strong heating is required for the second. In both steps, the process HCl and water vapor are evolved from the reaction mass. "Salt Cake" prepared by such a process was found to have the following composition: 91.48% Na2SO4, 4.79% NaHSO4, 1.98% NaCl, 1.35% H2O and 0.4% HCl. The salt used in the process is dry and may be assumed to be pure NaCl. On a basis of 1000 kg of salt charged. Calculate:(a) weight of acid added, weight of salt cake formed and weight of each gas driven off(b) % conversion of NaCl in the first reaction and the degree of completion of the second reaction.arrow_forwardQ2: A mixture of gases content (50% methane, 30% ethane, 20% propane) feeds a combustion chamber. Calculate the amount of air feed at 30% excess?arrow_forward
- Sulfur reacts with oxygen to produce sulfur trioxide. If 58.7 mol/h of each reactant enters the reactor, and the reaction proceeds such that the fractional conversion of the limiting reactant is 0.28, how much of the excess reactant (mol/h) exits the reactor?arrow_forwardA gases fuel composed of 80 mol % ethane (C;Hg) and 20 mol% N, is burned with 20% excess air. 60% of the ethane goes to CO, and the rest are goining to CO. calculate: a. The molal ratio of air input to the fuel burned. b. The orsat analysis of the flue gas. c. The molal ratio of water vapour produced to the fuel burned.arrow_forward11.7 mole dichloromethane (CH2Cl2) enter a reactor with 21.6 mole hydrogen (H2) and 37.5 mole oxygen (O2). The following reaction takes place: CH2Cl2 + H2 + 3/2 O2 → COCl2 + 2 H2O 6.4 mole of H2O are produced. Calculate the extent of the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The