
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
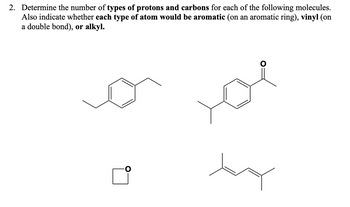
Transcribed Image Text:2. Determine the number of types of protons and carbons for each of the following molecules.
Also indicate whether each type of atom would be aromatic (on an aromatic ring), vinyl (on
a double bond), or alkyl.
by
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine if the compound is aromatic, non-aromatic, or antiaromatic.arrow_forwardDraw the HOMO and LUMO for the given molecule. Explain how bonds would change as you excite this molecule with UV/ Visible light. Be specific.arrow_forwardmass number for the hafnium isotope with 106 neutrons Express your answer as an integer.arrow_forward
- How do I draw this resonance structure?arrow_forwardData for Problem 2 ZOO 180 150 120 140 100 80 770 дом -8 60 40 20 10 9 a 5 015 100 80 Relative Intensity 8 ส 20 20 40 50 ppm septet 3 2 60 70 80 90 100 m/zarrow_forwardhow do molecules of octocrylene interact with each other to form octocrylene? cite with reference pls.arrow_forward
- Which c. ..se structures shows how the pictured molecule will look after it is flipped over horizontally, as illustrated? он flip OH H H. OH он H. ..l OH none of these но Note: Only check the boxes of structures that correspond to a single horizontal flip of the original structure. Ignore structures that have been rotated or flipped multiple times or in other ways.arrow_forwardDraw both resonance structures of the anion formed by the reaction of the most acidic C-H bond of the compound below with base. • Include all valence lone pairs in your answer. . For structures having different hydrogens of comparable acidity, assume that the reaction occurs at the less-substituted carbon. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the symbol from the drop-down menu. 0- O Bi % 5 T G ▾ 99-85 A 6 SCH₂CH3 ChemDoodleⓇ Cengage Learning Cengage Technical Support Y H F6 On [F & 7 O U J F7 * 8 PrtScn | K Home 9 83°F Sunny O L A End 0 F10 P IN Previous Next> C 4x PgUp F11 J Save and Exit 9:38 AM 7/18/2022 PgDn + = F12 80arrow_forwardUse the dropdown menu to answer the following questions about the structures (1-4) shown. 1 2 3 Are 1 and 4 resonance structures? choose your answer... Are 1 and 3 resonance structures? choose your answer... The most stable resonance structure is choose your answer...arrow_forward
- I need help with this table because I struggle all of themarrow_forward1. State whether each of the following is a primary, secondary, or tertiary halide. a) CH, b) Br. c) CH,CH,CHCH, Farrow_forwardThe structure below has an unusually large dipole. A skeletal structure with a cation is provided below. Add double bonds and/or lone pair electrons to draw an aromatic resonance structure that explains the dipole. Include any additional charges.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY