
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
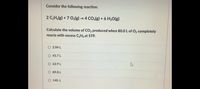
Transcribed Image Text:Consider the following reaction:
2 C,H,(g) + 7 O;(g) → 4 CO,(g) + 6 H,O(g)
Calculate the volume of CO2 produced when 80.0 L of O2 completely
reacts with excess C2Hgat STP.
O 2.04 L
O 45.7 L
O 62.9 L
O 89.8 L
O 140. L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2HCl(aq)+ Mg(s) —> MgCl2 (aq) + H2(g)arrow_forwardConsider the complete combustion of glucose (C6H12O6) with O2 and calculate the moles of CO2produced when 1.02 g of glucose is reacted with 25 mL of O2 at body temperature (37 ºC) and 0.970 atm. C6H12O6 + 6 O2 (g) → 6 CO2 (g) + 6 H2O a. 0.95 b. 0.0022 c. 9.53 x 10-4 d. 6.33 x 10-2 e. 5.67 x 10-3arrow_forwardHow many moles of oxygen gas are produced when 6.44 moles of water are produced? 2H2O2 (l) --> O2 (g) + 2H2O (l) (balancedarrow_forward
- Carry only 1 decimal point on all atomic weights (X.X). What is the mole fraction of O2 in a mixture that is 283.6 g N2, 145.1 g O2, and 5.111 g CO2?arrow_forward2 H3PO4(s) --> 3 H2(g) + 2 P(s) + 4 O2(g) Using the reaction above, show step by step how many liters of oxygen can be created if I start with 0.7156 grams of H3PO4 solid at STP?arrow_forwardPlease answerarrow_forward
- 4 NH3 (g) + 5 O2 (g) →→ 4 NO (g) + 6 H2O (l) What is the mole-mole factor (ratio) of H2O to NH3?arrow_forward1. Determine the Empirical Formulas from the % Composition data. Compound A: Compound A has a % composition (by mass) of 59.96% C, 26.62% O, and 13.42% H Compound A effuses 1.478 times faster than Xenon gas Compound B: Compound B has a % composition (by mass) of 62.04% C, 27.55% O, and 10.41% H Compound B effuses 1.063 times faster than Xenon gasarrow_forwardFor NH3 (g) + O2 (g) ---> NO (g) + H2O (g), balance the equation and find the number of liters of O2 (g) that can react with 1.5L of NH3 (g) if the gases are at the same temperature and pressure.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY