
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Question 19
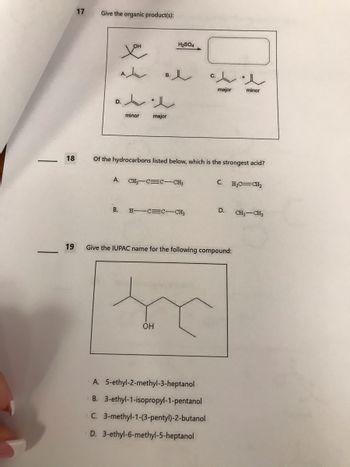
Transcribed Image Text:18
19
17
Give the organic product(s):
D.
you
A.
B.
t
minor
B.
e
major
H₂SO4
Of the hydrocarbons listed below, which is the strongest acid?
CH3 C C CH3
H-CECCH3
OH
major
A. 5-ethyl-2-methyl-3-heptanol
B. 3-ethyl-1-isopropyl-1-pentanol
C. 3-methyl-1-(3-pentyl)-2-butanol
D. 3-ethyl-6-methyl-5-heptanol
C.
t
minor
Give the IUPAC name for the following compound:
H₂C=CH₂
D. CH3 CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 21. Water goes through a chemical change when it evaporates into a gas. Group of answer choices a) True b) Falsearrow_forwardAlcohol evaporating is an example of what? Group of answer choices A chemical change A physical changearrow_forwardFill in the blank. Gravimetric analysis relies heavily on the principle of______ a. Conservation of energy b. Conservation of mass c. Constant composition d. Definite proportionsarrow_forward
- Round 0.221 to one sig. Figarrow_forwardCla Unit 1 Review Answer KEY- Goo X it 1 Quiz #4 Properties of MathX cl.castlelearning.com/Review/CLO/Student/Assignment/Questi Home - Hudl M East Rochest Quizlet Live Quizlet schools.org bookmarks Unit 1 Quiz #4 Pro 1 of 10 Question 1 Review Which statement describes a chemical property of hydrogen gas? 1. Hydrogen gas burns in air. 2. Hydrogen gas is colorless. 3. Hydrogen gas has a density of 0.000 09 g/cm at STP. 4. Hydrogen gas has a boiling point of 20. K at standard pre Standardarrow_forwardWhich is a property of a nonmetal? A. Poor conductor of electricity B. Solid at room temperature C. Shininess D. Good conductor of heatarrow_forward
- Part II Graham's Law (average speed and molar mass) - Submicroscopic View Your observation 1. The speed of the particles is (circle one) a. Constant b. Variable C. same 2. The particles move in a between collisions. a. straight –line b. curved path 3. The speed of the particles changes following the collision with a. another particle b. the wall of the container c. both the wall of the container and another particle. Use the Track function explained earlier to help answer this question. 4. Collisions between particles or the walls of the container are elastic or not elastic. 5. The particles attractions to other particles. а. еxperience b. do not experience 6. The RMS speed of helium is as the mole of helium changes. a. constant b. variable 7. Record the RMS speeds of different gases in the following table. Table 1. RMS speed of gases Gas Не Ne Ar Molar mass g/mol RMS speed m/s 8. Is your observation of RMS speeds consistent the Graham's law of effusion, which states that rates of…arrow_forwardDiscuss the language demands included in the lesson on mixing substances in a 5th-grade classroom by Identifying key vocabulary terms students must know in order to master the learning objectives for the lesson based on the below standard and learning objective. Standard Conduct an investigation to determine whether the mixing of two or more substances results in new substances. Learning Objective Given a variety of substances and safety equipment in a classroom setting, students will be able to conduct an experiment to mix two or more substances and observe the results. They will be able to correctly identify whether a new substance has been formed as a result of the mixing, based on observable changes such as color change, formation of a precipitate, or change in temperature, with at least 80% accuracy.arrow_forwardSuppose that you heated the hydrated copper (II) sulphate in a test tube, instead of a beaker. How might this affect your results? 4 MAY 19 ... MacBook Pro & * %23 $ 2 3 4 5 6 7 E Y U G H J K C V M MOSISO command option command レーarrow_forward
- 7. Match each characteristic as either a physical or chemical property. Question 7 options: boiling point pH malleability heat of vaporization 1. chemical 2. physicalarrow_forwardI need help with the blank tables on the page as well as the question on the bottomarrow_forwardan introduction on a research scientistarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY