
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
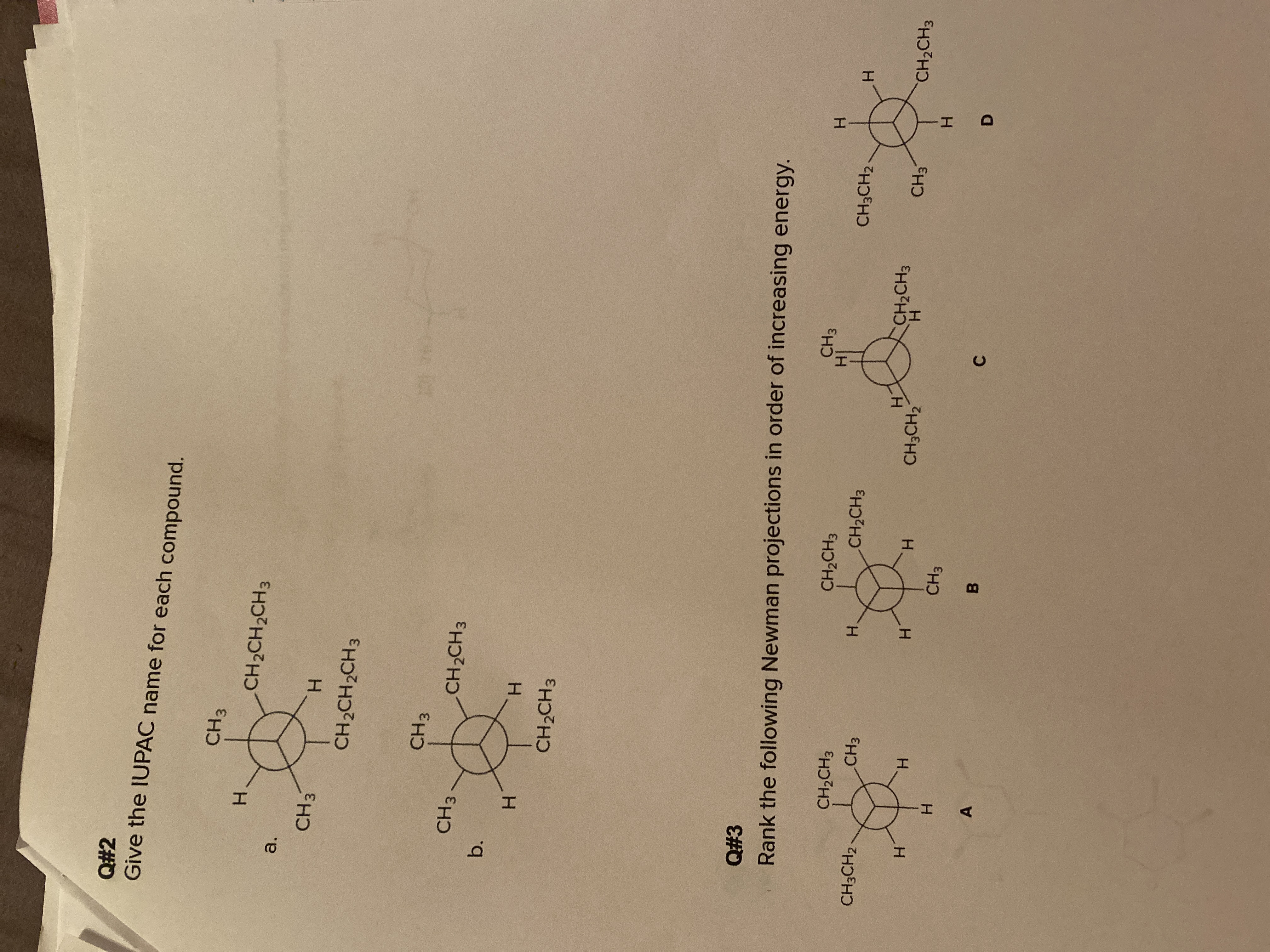
What is questions #2-b and questions # 3?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The combined ages of three dogs is 24 years. Dog B is five years less than twice the age of dog A. Dog C is one year more than one half the age of Dog A. How old is dog C? Question 6 options: 11 years 5 years 8 yearsarrow_forwardbleach container costs $5.98 and it has a volume of 2.4 ml what is the cost per gallon? The density of bleach solution is 1.05g/mlarrow_forwardCelsius scale water freezes On the Celsius scale, water freezes at 32 degrees. True or False True Falsearrow_forward
- give a particulate-level explanation of why ice (solid water) is less dense than liquid water?arrow_forwardQuestion 20 Which of the following correctly describes an energy transformation that can be observed in an everyday situation? A Gasoline burns and powers a car engine when the bonds are broken between the molecules and the free electrons flow. This is a transformation between electromagnetic energy and chemical energy. B Ice in soda glass melts when it is placed in sunlight on a window sill. Radiant energy from the sun is absorbed by the water molecules, which increases the speed that the molecules move. This is a transformation between light energy and kinetic energy. C A pot of water is heated by a stove as the heat energy moves from the stove top to the metal of the pot. This is a transformation between mechanical energy and thermal energy. D The sun is powered by the fusion of large atomic nuclei that creates heat and light for the solar system. This is a transformation between chemical energy and light energy.arrow_forwardWhich of the following is a pure substance that can be broken down into simpler substances by chemical means? Solution Compound Heterogeneous mixturearrow_forward
- Gasoline is composed of a variety of different liquid hydrocarbons, which do not separate as time passes. Gasoline is an example of a: A) heterogeneous mixture B) Chemical compound C) Chemical element D) Solutionarrow_forward= 24 = 25 = 26 27 = 28 29 = 30 F 31 = 32 = 33 34 35 36 Calculate the amount of heat needed to boil 14.8 g of ethanol (CH,CH,OH), beginning from a temperature of -26.4 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. dh Submit Assignment Continue MacBook Air A. FI F12 F9 F10 FIC FB 000 D00 F4 F7 20 F6 F5 esc F3 F1 & deleto $ %D @ 7 8 2 3 4 5arrow_forwardUse the image to answer the following question. ННННН НННН ННННН H-C-C-C-C-Ç-C-C-C-C-Ç-C-C-C-C-C HH HHHHHHHHHHHH OH An engineer is developing a new material that would allow electricity to flow. Which of the following materials would an engineer choose and why? O A. Copper, because it contains ions that have the ability to conduct electricity. O B. Carbon, because it is a good insulator. O C. Copper, because it is a good insulator. D. Carbon, because it contains ions that have the ability to conduct electricity.arrow_forward
- Part A- Does a liquid release energy or absorbs energy when it changes to a solid?(think of when water changes to ice). A-release energy B-absorbs energy Part B- Absolute zero is the freezing point of water- 0 degrees Celsius and 0 degrees farenheight A-True B-Falsearrow_forwardEvery year Every second (1 year 365 days).arrow_forwardConsider the two spheres shown here, one made of silver and the other of aluminum. The spheres are dropped from a height of 1.7 m. Composition - aluminum Density= 2.70 g/cm³ Volume 196 cm³ Composition - silver Density 10.49 g/cm³ Volume=196 cm³ What is the kinetic energy of the silver sphere at the moment it hits the ground? (Assume that energy is conserved during the fall and that 100%% of the sphere's initial potential energy is converted to kinetic energy the time impact occurs.) Express your answer to two significant figures and include the appropriate units. Ek = Value Unitsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY