
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
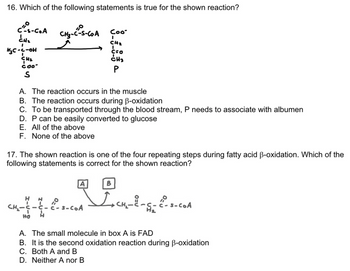
Transcribed Image Text:16. Which of the following statements is true for the shown reaction?
C²-S-CoA CH₂-C-S-COA çoo-
CH₂
CH ₂
H₂C-C-OH
CH₂
COO
S
A. The reaction occurs in the muscle
B. The reaction occurs during B-oxidation
C. To be transported through the blood stream, P needs to associate with albumen
D. P can be easily converted to glucose
E. All of the above
F. None of the above
17. The shown reaction is one of the four repeating steps during fatty acid ß-oxidation. Which of the
following statements is correct for the shown reaction?
H
C=O
CH3
P
HO H
A
s-CoA
B
I+CH₂-8-5-C²-S-COA
A. The small molecule in box A is FAD
B. It is the second oxidation reaction during B-oxidation
C. Both A and B
D. Neither A nor B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- Give detailed Solution with explanation needed..don't give Handwritten answerarrow_forward2. The mechanism of HMG-CoA reducatse enzyme activity involves several stages. For the catalytic reaction to proceed, another substrate known as NADPH, which acts as a reducing agent is required. The kinetics of enzyme activity with NADPH at non-limiting amounts of HMG-CoA in the absence or presence of compactin is shown below. a) Based on the description above, which of the six types of enzymes does HMG-CoA reductase belong to? b) What is the Km for the reaction with no inhibitor present? Do NOT forget the units. Answers can be expressed either as a fraction or in decimals. c) What happens the measured Km with increasing amounts of compactin? (select one) increases decreases does not change d) What happens to the measured Vmax with increasing amounts of compactin? (select one) increases decreases does not change e) Based on your answers above, what type of inhibitor is compactin relative to its effects on enzyme activity NADPH? with -60 -40 1/V, (nanomoles/min)-1 -20 0.2 0.18 0.16…arrow_forward3. Palmitate is radiolabeled at the ¹¹ position using ¹4C. This is added to a liver homogenate. Trace the flow of the radiolabeled carbon as the palmitate is catabolized and then used for ketone body production. If a molecule can have the radiolabel in two different positions, make sure to show both possibilities. Present your work as a progression through the catabolic pathway, showing the product(s) from every round of B-oxidation and the subsequent steps to ketone body production that contain the radiolabel. Make sure each step also shows any needed cofactors and/or enzymes.arrow_forward
- The pentose phosphate shunt is likely to be active when: a AMP levels are high b the organism has not had a meal for a long time. c an organism is growing d fatty acid beta-oxidation is active.arrow_forwardThe reaction below catalyzed by phosphoglycerate mutase is, 3-phosphoglycerate 2-phosphoglycerate an isomerization. A. В. a group transfer reaction. a reversed aldol condenstion. C. Op an oxidation reduction reaction. E. Cand Darrow_forward6. Lovastatin (see picture), is a potent competitive inhibitor of HMG-COA reductase (B-hydroxy- B-methylglutaryl-COA reductase). In addition, the synthetic compound, Terbinafine (see picture), is a potent inhibitor of squalene monooxygenase. Both compounds could be used to treat hypercholesterolemia, a genetic disorder where the patient synthesizes too much cholesterol regardless of dietary intake of cholesterol. a. Predict and explain the effect of these drug on serum cholesterol levels in humans. b. Based on squalene monooxygenase's role in synthesizing sterols, explain why terbinafine is marketed as an anti-fungal agent, and not for hypercholesterolemia.arrow_forward
- 21. How many carbons are removed from fatty acyl CoA in one turn of B-oxidation spiral? E. 6 22. B-oxidation of fatty acids is promoted by which of the followings? D. Acetyl CoA A. 1 С. З D. 4 B. 2 A. ATP B. NAD+ C. FADH2 E. Propionyl CoAarrow_forward14. Which of the following statements is true for the shown reaction? P-O-CH₂ HOO-P ក្រុង D P-O-CH₂ OH MOTHE OH ночно HO OH B A A. Stimulation of the reaction slows down glycolysis B. The reaction is the rate-limiting step of gluconeogenesis C. Both A and B D. Neither A nor Barrow_forward9. Which of the following statements is/are correct for the shown reaction? A CHg-c-s-col + CO - C-CH₂-C-S-COA A. The reaction occurs in the mitochondria B. The small molecule in box a is GTP C. The reaction is a committed step for fatty acid catabolism D. The reaction requires biotin E. None of the abovearrow_forward
- 10. Which of the following processes involve release of carbon dioxide? (1) B-oxidation (2) Citric acid cycle (3) Activation of acetyl CoA to form malonyl CoA (4) Pentose phosphate pathway A. (1) and (2) only B. (1) and (3) only C. (2) and (4) only D. (1), (3) and (4) only E. (1), (2), (3) and (4)arrow_forward2. Draw the reactions catabolizing the amino acid valine into isobutryl-CoA, specifically one transamination reaction (for which you do not need to show the mechanism) and one dehydrogenation reaction (which is analogous to the pyruvate dehydrogenase reaction mechanism, and for which you need to show the mechanism using arrow pushing and including the relevant cofactors). Note: Beyond the importance of these reactions for human health, this pathway is used industrially in the production of isobutanol, an important biofuel. NH3 SCOA valine isobutyryl-CoAarrow_forward2. In contrast to the toxicity of 2-fluoro-citrate, L-(2-fluoro-)succinate is not an inhibitor of succinate dehydrogenase and the product 2-fluoro-fumarate is converted by fumarate hydratase to L-(2-fluoro-2- hydroxy)malate that breaks down to oxaloacetate. (a) the FAD coenzyme of succinate dehydrogenase as the normal substrate? Write the reaction with structural formulas to illustrate your answer. Does the oxidation of 2-fluoro-succinate yield the same number of reducing equivalents to (b) droxy)malate, which breaks down spontaneously to oxaloacetate. Write the equation with structural formulas. 2-fluoro-fumarate is a substrate of fumarate hydratase forming only L-(2-fluoro-2-hy- Oxaloacetate has been shown to be a very po- (c) tent inhibitor of succinate dehydrogenase. For the enzyme 3 isolated from cardiac muscle the Kj value is ~4 x 10-6M, as shown in the L-B plot on the right. Yet, this inhibition is never discussed in biochemistry textbooks. From your reading in the textbook,…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON