
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please solve a question quickly
I need this Answer at half time Quickly. Please
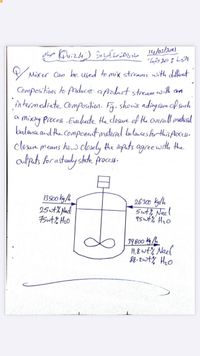
Transcribed Image Text:V Mixer Can be used to mix streams with diffrat
Compositions to Priduce: afioduet stream wwith am
întermediate omposihion. Fig, shows adiag ram of such
Mixiny Process Evulite the closure of the Ouverall madnal
balane and the Component materal bulances for this Aocesso
closue means how closely the mputs agreewith the
aulputs for asteady shade process.
26300 kglh
25t% Nad
5ut% Nael
95wf% H2o
39800 4/4
1,8 wH% Nael
88.2wt% Hho
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Your Question :Your Question :Your Question :Your Question :help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!arrow_forwardCanvas XC 2 X CO Summer 2024 Home Discussions Help Center The best grade will count as your homework grade. hw_Condensation al Ch E ndar box D Grades People Syllabus Quizzes Modules Collaborations Cisco Webex Library Resources Chat Score: 0/6 Answered: 0/1 12 Question 1 Microsoft OneDrive Class Notebook istory Microsoft Teams classes 凹 Studio Help K Microsoft Teams meetings University Syllabus Office 365 Adobe Creative Cloud Bulldog Bundle Digital Materials Credentials Steam is used on the shell-side of a vertical shell/tube exchanger to heat water. Given the following: Do = 0.75,in There are 100 tubes 14 ft long Condensing temperature 280 F and Heat of Vaporization = 1050 btu/lb The tube wall temperature can be assumed to be 195 F What is the condensate film temperature, F? 216.25 Also given: cond viscosity at 216.25 0.68077625 lb/ft-h cond k at 216.25-0.394 btu/ft-h-F cond cp at 216.25 -1.0 btu/lb-F cond density at 216.25 = 58.2 lb/ft3 What is the condensate prandtl number, Pr?…arrow_forwardin packed towers experiment, The Ergun equation is a fine starting point for typical use in helping size/design columns in a variety of applications, however it makes numerous assumptions and approximations in its derivation. Can you name and explain (why) 2 regimes of operation where you clearly think the Ergun equation (at least the one we use in this class) would not be appropriate to use in column design?arrow_forward
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The