
Concept explainers
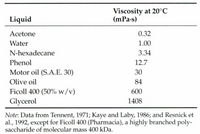
Consider the data table below. Based on the interpretation of the data, select all of the following that are true.
Screen Shot 2020-11-24 at 9.49.36 AM.png
Group of answer choices
Of the liquids given, Glycerol has the strongest intermolecular forces
Of the liquids given, Acetone has the strongest intermolecular forces
You must have the chemical structures to know anything about the intermolecular forces
From this data, motor oil likely has a higher vapor pressure than olive oil
From this data, motor oil likely has a lower vapor pressure than olive oil
From this data, hexadecane likely has a higher boiling point than water
From this data, hexadecane likely has a lower boiling point than water
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

- Fluid mechanicsarrow_forwardShort answers: (a) How do you determine if you should consider both natural and forced convection? Use equations to explain your logic.arrow_forwardQ_ The product of poly ethylene contains twelve molecules, four of the molecules have Dp=220. three of the molecules have Dp- 370. five of the molecules have Dp=110.Calculate the Mn, Mv, K, and (relative and specific viscosity). If time of descent solvent and Polymer (10. 15 sec) respectively, MWD= 0.88. a= 1?arrow_forward
- This problem is (11.16) from a book "Thermodynamics and Statistical Mechanics An Integrated Approach by M. Scott Shell"arrow_forwardDerive the equation for each of the step mentionedarrow_forwardEXPERIMENT: EFFECT OF REGULATING REFLUX RATIO ON TOPPRODUCT COMPOSITION IN DISTILLATION PROCESS OBJECTIVES : i) To perform stage distillation by separating ethanol and water on different tray ii) To study mass fraction for ethanol and water on different tray In summary, the fundamental procedure in distillation was performed clearly. The pure product of ethanol and water were collected on top product valve and bottom product valve respectively. The refractive index for ethanol and water were observed using the refractometer two times to obtain the reading for the first tray and forth tray. QUESTION: PLEASE MAKE AN INTRODUCTION PARAGRAPH BASED ON THIS EXPERIMENT.arrow_forward
- Times New ... A Questions 1. What causes the liquid to move one direction or the other in the U-shaped part of the apparatus? You may wish to read the explanation found on the experiment webpage by clicking on "Isoteniscope Method" inside the red box (the explanation will appear in a pop-up window). 2. Does it matter in this experiment how much of the liquid phase was present? Explain your answer. 3. a. Look at the value of the vapor pressure at approximately 35°C for both ethanol and cyclohexane in your data Record these values here.arrow_forwardFor chemical engineers if Its correct I will upvote Derive a well established relationship between Asorbance and Concnetration of solution using Beer- Lambert law.Please show how to use integration and Differentiation on intensity of radition.arrow_forwardWhat is the volumetric flow rate in L/h of Feed? What are the split fraction SF and split ratios SR for Propylene?arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





