
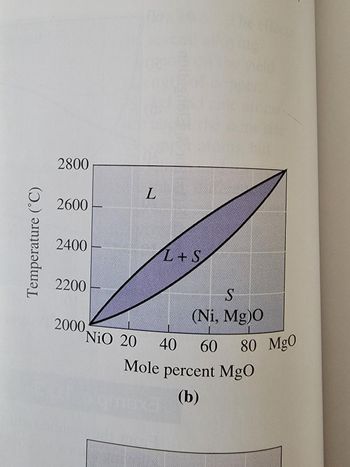
A NiO-20 mol% MgO ceramic is heated to 2200 °C. Determine
(a) the composition of the solid and liquid phases in both mol% and wt%.
(b) the amount of each phase in mol% and wt%.
(c) assuming the the density of the solid is 6.32 g/cm3 and that of the liquid is 7.14 g/cm3, determine the amount of each phase in vol%.
See attached image
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

I apologize upfront, but I do not understand the solutions here. I cannot follow along due to the way it is typed. I've tried writing it out many times and never come to the same answers as you. Is it possible to get these solutions hand written?
I apologize upfront, but I do not understand the solutions here. I cannot follow along due to the way it is typed. I've tried writing it out many times and never come to the same answers as you. Is it possible to get these solutions hand written?
- NEED IT UNTIL 5:15PM Estimate the convective heat-transfer coefficient for natural convection from a horizontalsteam pipe. The outside surface temperature of the insulated pipe is 80°C. Thesurrounding air temperature is 25°C. The outside diameter of the insulated pipe is 10 cm. please help me if my answer is wrong.arrow_forward3arrow_forwardcalculate the boiling point of a solution of 400.0 g of ethylene glycol dissolved in 400 g of waterarrow_forward
- Nitrogen dioxide, a pollutant in the atmosphere, can combine with water to form nitric acid. One of the possible reactions is shown here. Calculate ΔG° and Kp for this reaction at 25 °C and comment on the spontaneity of the reaction.3 NO2( g) + H2O(l )------->2 HNO3(aq) + NO( g)arrow_forwardThe results of air temperature measurement obtained a dry bulb temperature of 37 ° C and a wet bulb temperature of 27.5 ° C. Using a psychrometric chart, determine the properties of the air as follows:a. RH:%b. Water content: kg water / kg airc. Specific volume: m3 / kgd. Enthalpy: kJ / kge. Condensation temperature: ° CIf the air is within 162 m3 space, determinef. Air weight (dry air and water vapor): kgg. the amount of water content in the space: kgarrow_forwardA rectifying column consists of a tube arranged vertically and supplied at the bottom with a mixture of benzene and toluene as vapor. At the top, a condenser returns some of the product as a reflux which flows in a thin film down the inner wall of the tube. At one point in the column, the vapor contains 60 mol% benzene and the adjacent liquid reflux contains 51 mol% benzene. The temperature at this point is 92°C. Assuming the diffusional resistance to vapor transfer to be equivalent to the diffusional resistance of a štagnant vapor layer 0.5 mm thick, calculate the rate of interchange of benzene and toluene between vapor and liquid. The molar latent heats of the two materials can be taken as equal. The vapour pressure of toluene at 92°C is 44.0 kN/m2 and the diffusivity of the vapors is 0.053 cm?/s.arrow_forward
- The air at a dry ball temperature of 80 oC and 4 % RH is passed over the cooling coil so that the air dry ball temperature becomes 35 oC. how much heat is released from the air in the process? = (kJ / kg)arrow_forwardA saturated solution containing 150 kilograms of sodium sulfate (mol. wt. = 142) in 600 kilograms of water is fed to Swenson-Walker crystallizer. The solubility of sodium sulfate at 10 °C is 9 kilogram of anhydrous salt per 100 kilogram of water, and the deposited crystals consist of the decahydrate (mol. Wt. = 322). Crystallization is by cooling at 10 °C. If 2% of the water was lost by evaporation during the cooling process, the percent (%) yield in the crystallizer is Blank 1%. **(Express your answer to two (2) decimal places. Do not put units on your answer)**arrow_forwardWhat are the relative humidity and dew point temperature of 1 kilogram of 25 degree Celsius air that contains 10 grams of water vapor?arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





