
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:red
140
120
d out of
100
80
g question
60
40
20
a
- 20
20
40
100
120
Time (min)
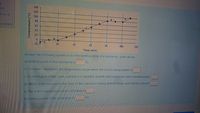
Answer the following questions for the heating curve of a substance, given above.
a) Melting point of the substance is
°C.
b) The letter represents the temperature range where the solid is being heated is
C) In which part of the curve, substance is available in both solid and liquid state simultaneously?
d) Which letter represents the state of the substance having definite shape and definite volume?
e) The letter represents process of boiling is
) Freezing point of the substance is C.
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 8 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- substance A has normal melting point of -25.0 degrees celsius, an enthalpy of fuision of 1200 J/g; specific heat for solid is 3.00 J/g degrees celsius and for the liquid id 6.20 J/g degrees celsius. calculate the energy required to change 150 grams of A from a solid at -40.0 degrees celsuis to a liquid atarrow_forward1. What set rigid solids apart from amorphous solids, like glass? Talk briefly. Please answer with full detailarrow_forwardhow much heat will it take to raise temperature of 10g of ice from -30c to -20carrow_forward
- A 41.30 g piece of solid acetone has a temperature of -119.2°C. How much heat is involved in converting it to a liquid with a temperature of -94.0°C? Formula: C3H6OMelting Point: -94.0°CBoiling Point: 56.0°CDensity of liquid: 0.791 g/mLHeat of Fusion: 98.14 J/gHeat of vaporization: 538.9 J/gSpecific heat capacity (solid): 1.653 J/g°CSpecific heat capacity (liquid): 2.161 J/g°CSpecific heat capacity (gas): 1.291 J/g°Carrow_forwardA pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: 12 230 temperature (°C) 210. 190. 170 150.- 130. 0. 10. What is the melting point of X ? Use this graph to answer the following questions: 30. heat added (kJ/mol) What phase (physical state) of X would you expect to find in the flask after. 17 kJ/mol of heat has been added? 40 50. 0°C (check all that apply) Osolic O liquid Ogas 60 olo Ararrow_forwardConsider the following phase diagram. Phase diagram for compound A 1000 2 760 3 50 10 -25 25 100 150 Temperature, °C (not to scale) Which red dot represents the melting point of this compound at 760 torr? [ Select ] What is the approximate melting temperature for this substance at 760 torr? [ Select ] Which red dot represents the boiling point of this compound at 760 torr? [ Select ] What is the approximate boiling temperature for this substance at 760 torr? [ Select ] Which red dot represents the triple point for this substance? [ Select ] |What is the approximate temperature of the triple point? [ Select ] Pressure, torr (not to scale)arrow_forward
- From point A to point B, choose one or more of the following, gas is heated solid is heated solid is cooled freezing vaporization melting deposition sublimation liquid is heated gas is cooled liquid is cooled condensationarrow_forwardClassify the following phase changes as processes that require the input of energy, or as processes that have a net output of energy. Drag the appropriate items to their respective bins. > View Available Hint(s) Reset Help vaporizing condensing deposition subliming melting freezingarrow_forward= STATES OF MATTER Identifying phase transitions on a heating curve A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: temperature (°C) 170. 150. 130. 110. 90 70. 0. 10. What is the melting point of X ? Use this graph to answer the following questions: heat added (kJ/mol) What phase (physical state) of X would you expect to find in the flask after 5 kJ/mol of heat has been added? *C 30. X 40. (check all that apply) solid liquid gas 0/3 50.arrow_forward
- 3. A substance has a heat of vaporization of 28.90 kJ/mol. At -21 °C it has a vapor pressure of 137 mmHg. What is the temperature in °C when the vapor pressure is 14 mmHg? Round to the nearest whole number.arrow_forwardUse the observation in the first column to answer the question in the second column. observation question Which has the higher boiling point? O Substance E The enthalpy of vaporization of Substance E is smaller Substance F than that of Substance F. Neither, E and F have the same boiling point. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 36 °C, Substance C has a Substance C vapor pressure of 140. tor and Substance D has a vapor Substance D pressure of 130. torr. Neither, C and D have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 1 atm pressure, Substance A Substance A boils at -12. °C and Substance B Substance B boils at 1. °C. Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY