
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![Magnesium hydroxide will dissolve in an aqueous solution of ammonia and drive off
ammonia gas according to the following reaction:
Mg(OH)2(s) + 2 NH4*(aq) – Mg2*(aq) + 2 H20(1) + 2 NH3(g)
The standard enthalpies of formation for each of the species involved are:
Mg(OH)2(s) [-924.5 kJ/mol); NH4*(aq) [-132.5 kJ/mol]; Mg2*(aq) [-466.9 kJ/mol];
H20(1) [-285.8 kJ/mol]; NH3(g) [-46.11 kJ/mol].
What is the standard enthalpy of reaction for the magnesium hydroxide solvation
process as written above?
-294.1 kJ/mol
+58.8 kJ/mol
+258.2 kJ/mol
-58.8 kJ/mol
-408.1 kJ/mol
-258.2 kJ/mol](https://content.bartleby.com/qna-images/question/8294c3eb-eeac-4c99-a406-a76a307762ce/57e70ad6-3c84-465b-8887-57138c20b58c/9le9u4l_thumbnail.jpeg)
Transcribed Image Text:Magnesium hydroxide will dissolve in an aqueous solution of ammonia and drive off
ammonia gas according to the following reaction:
Mg(OH)2(s) + 2 NH4*(aq) – Mg2*(aq) + 2 H20(1) + 2 NH3(g)
The standard enthalpies of formation for each of the species involved are:
Mg(OH)2(s) [-924.5 kJ/mol); NH4*(aq) [-132.5 kJ/mol]; Mg2*(aq) [-466.9 kJ/mol];
H20(1) [-285.8 kJ/mol]; NH3(g) [-46.11 kJ/mol].
What is the standard enthalpy of reaction for the magnesium hydroxide solvation
process as written above?
-294.1 kJ/mol
+58.8 kJ/mol
+258.2 kJ/mol
-58.8 kJ/mol
-408.1 kJ/mol
-258.2 kJ/mol
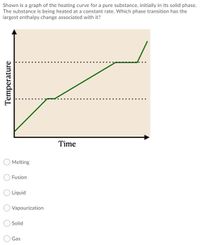
Transcribed Image Text:Shown is a graph of the heating curve for a pure substance, initially in its solid phase.
The substance is being heated at a constant rate. Which phase transition has the
largest enthalpy change associated with it?
Time
Melting
Fusion
O Liquid
Vapourization
O Solid
Gas
Temperature
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the enthalpy change that occurs when 3.20 g of SnCl4 (1) reacts with excess H₂O(l) to form SnO₂ (s) and HCl(aq). Use the following enthalpies of formation: AfH (kJ/mol) Compound HCl(aq) H₂O(l) SnCl4 (l) SnO₂ (s) Enthalpy change = -167.159 -285.83 -511.3 -577.63 kJarrow_forwarda) Calculate the enthalpy variation produced in the butane combustion reaction under standard conditions.b) what amount of heat will be released in the complete combustion of the 12kg of butane contained in a cylinder dH standard CO2 (g) = -393kJ / mol, H2O (l) = -286kJ / mol, C4H10 (g) = -125kJ / molarrow_forwardCalculate the change of enthalpy for the reaction 2AI (s) + 3C12 (g) --> 2AICI3 (s) from the following reactions: Reaction 1: 2AI (s) + 6HCI (aq) --> 2AICI3 (aq) + 3H2 (g) Change in enthalpy: -1049 kJ Reaction 2: HCI (g) --> HCI (aq) Change in enthalpy: -74.8 kJ/mol Reaction 3: H2 (g) + Cl2 (g) --> 2HCI (g) Change in enthalpy: -1845. kļ/mol Reaction 4: AICI3 (s) --> AICI3 (aq) Change in enthalpy: -323. kJ/mol Include the following: • The numerical answer with correct units. • State which reactions, if any, you had to "Flip". • State which reactions you had to multiply, if any, to get the correct amount of the compound.arrow_forward
- Nitric acid, HNO3, reacts with potassium hydroxide as follows: HNO3(aq) + KOH(aq) ⟹ KNO3(aq) + H2O(l) A student places 55.0mL of 1.3M HNO3 in a coffee cup calorimeter, and noted that the temperature was 23.5oC. 55mL of 1.3M KOH also at 23.5oC was added. After quickly stirring with a thermometer, the temperature rose to 31.8oC. Calculate the heat generated by the reaction.arrow_forwarda) What is the standard enthalpy of reaction per mole of either HCl or NaOH when 50.00 cm3 of 0.1 moldm-3 of hydrochloric acid (HCl) and 50.00 cm3 of 0.1 moldm-3 of sodium hydroxide (NaOH) are mixed? According to your simulation, the temperature rose by 0.68 oC. (Density of water = 1gcm-3, Specific heat capacity of water = 4.18 J g-1 oC-1) Show your working below:arrow_forwardCalculate the amount of heat released (absolute value) when 0.700 kg of liquid ethanol is burned in excess oxygen to make carbon dioxide and liquid water. The enthalpies of formation of the products and reactants are:CO2(g): -393.51 kJ/molH2O(l): -285.83 kJ/molO2(g): 0 kJ/molC2H5OH(l): -276.0 kJ/molarrow_forward
- Which of the following reactions occuring at 25° C defines the standard enthalpy of formation (TriangleHf) of liquid water? - 2 H (g) + O (g) -> H2O (l) - H2O(g) + O (g) -> H2O (l) - H2 (g) + ½O2 (g) -> H2O (l) - 2 H2 (g) + O2 (g) -> 2 H2O (l)arrow_forwardThe reaction between hydrogen and oxygen to yield water vapor has AH" = -1843 2H₂(g) + O₂(g) 2H₂O(g)AH <=-484kJ Part A What is the enthalpy change in kilojoules when 0.70 mol of H₂ reacts with 0.35 mol of O, to produce 0.70 mol of H₂ at constant pressure of 1.00 atm? Express your answer as a whole number. AH = Submit Part B W= Submit 1957 ΑΣΦ How much PV work is done in kilojoules for this reaction at constant pressure of 1.00 atm if the volume change is-9-2 L? Express your answer using two significant figures. 100 ΑΣΦΑ Part C Request Answer Request Answer kJ What is the value of AE for this reaction in kilojoules?arrow_forwardThe standard heat of formation, AH;, is defined as the enthalpy change for the formation of one mole of Part B substance from its constituent elements in their standard states. Thus, elements in their standard states have AH: = 0. Heat of formation values can be used The combustion of ethene, C2H4, occurs via the reaction to calculate the enthalpy change of any reaction. C2H4 (g) + 302 (g)→2CO2(g) + 2H20(g) Consider, for example, the reaction with heat of formation values given by the following table: 2NΟ (5) + Ο, (s) 2ΝO, (g) ΔΗρ (kJ/mol) Substance with heat of formation values given by the following table: C2H4 (g) 52.47 CO2 (g) -393.5 Substance (kJ/mol) H2O(g) -241.8 NO(g) 90.2 Calculate the enthalpy for the combustion of ethene. 02 (g) Express your answer to four significant figures and include the appropriate units. NO2(g) 33.2 • View Available Hint(s) Then the heat of formation for the overall reaction is ΔΗ ΔΗ (products) -ΔΗ; (reactants ) [2(90.2) + 0] HA ? 2(33.2) -114 kJ/mol…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY