
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A. Provide a stepwise mechanism for the formation of nerolidyl pyrophosphate from
farnesylpyrophosphate
B. Provide a stepwise mechanism for the formation of carbocation 1 from nerolidyl
pyrophosphate. Number the backbone carbons of nerolidyl pyrophosphate from 1 to 11 as shown, and
include the carbon numbering in your structure of 1
C. Following from B, give an arrow-pushing mechanism to convert 1 to 2 and 2 to 3. Use the
backbone carbon numbering from 1 to indicate where carbon atoms ended up in 2 and 3
D. In addition to forming epi-cedrol, carbocation 3 gives three minor byproducts: a diastereomeric
alcohol and two
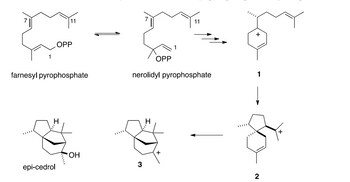
Transcribed Image Text:OPP
11
OPP
11
+
farnesyl pyrophosphate
nerolidyl pyrophosphate
1
I
epi-cedrol
OH
3
Ι
+
2
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 22-25 Draw the most predominant form of lysine at its isoelectric point.arrow_forward22-97 Gelatin is derived from collagen by denaturation. Is a gelatin dessert likely to be a good source of dietary protein?arrow_forward22-82 (Chemical Connections 22H) How does the fiberscope help to heal bleeding ulcers?arrow_forward
- 21-60 (Chemical Connections 21C) Why were Mark McGwire and Floyd Landis not given the same penalties for taking steroids in their sports?arrow_forward21-29 Name all the groups of complex lipids that contain ceramides.arrow_forward22-65 (a) What is the difference in the quaternary structure between fetal hemoglobin and adult hemoglobin? (b) Which can carry more oxygen? (c) What would the oxygen saturation curve of fetal hemoglobin look like compared to that of myoglobin and regular adult hemoglobin?arrow_forward
- 22-75 (Chemical Connections 22A) Why must some people avoid drinking diet sodas with Nutrasweet?arrow_forward22-48 How many amino acid residues in the A chain of insulin are the same in insulin from humans, cattle (bovine), hogs, and sheep?arrow_forwardi H₂N-CH-C-N-CH-C-N-CH-C-OH CH₂ i CHCH₂ CH₂ H H H + water HCI valine phenylalanine + glycinearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning