
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Determine the structure of the unknown using the data provided please, thank you.
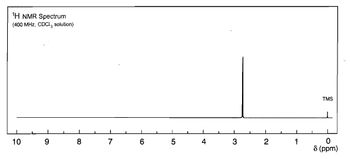
Transcribed Image Text:1H NMR Spectrum
(400 MHz, CDC3 solution)
10
9
8
1
7
Co
6
LO
5
4
3
TMS
2
1
1
0
8 (ppm)
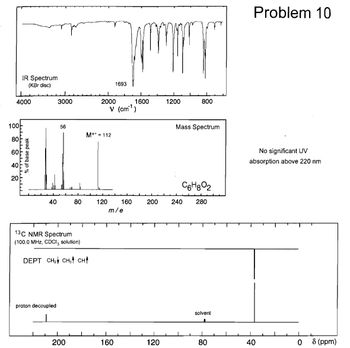
Transcribed Image Text:100
80
60
IR Spectrum
(KBr disc)
1693
4000
3000
2000
1600
1200
800
V (cm³)
% of base peak
יזיזיויויניויויויזיד
40
20
56
M+112
Problem 10
Mass Spectrum
No significant UV
absorption above 220 nm
C6H802
40
80
120
160
200
240
280
m/e
13C NMR Spectrum
(100.0 MHz, CDCI, solution)
DEPT CH2 CH3 CH↑
proton decoupled
solvent
200
160
120
80
40
40
0
8 (ppm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Part B Br Br Spell out the full name of the compound. Submit Request Answer Provide Feedback to searcharrow_forwardtab caps lock shift esc ર. Freeform File → + С ! 1 *; Aktiv Chemistry - F1 Q A Edit N app.aktiv.com Insert NO @ 2 * F2 W S Format Arrange NaH X + DMSO OH #3 X Br 80 F3 E D View Window $ 4 C a 49 a F4 R F Help % 5 £ FS T Problem 254 of 24 Atoms, Bonds and Rings 4. CI 6 G stv MacBook Air ♫ 9 F6 Y & 7 H Charges 8 OH Br F7 00* 00 Na 8 OH 12 C all DII FBarrow_forwardFor which kind of organic molecules are molecular modeling exercises most effective?arrow_forward
- What are names of these groups?arrow_forward- Google Se x + Online teaching and lea X G CuO chemical name - Google Se X _com/ilm/takeAssignment/takeCovalentActivity.do?locator-assignment-take on Classes q 3 D Sjuju_ 4 eq eq Not Visited req Ⓡ req Free Online Survey... Q Search formula = Home - Academia... Create Your Rubric... The Utility Experts ... A solution contains 7.44x10-3 M lead nitrate and 1.43x10-2 M calcium acetate. Solid sodium phosphate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? Submit Answer W Use the References to access important values if needed for this question. M B. What is the concentration of phosphate ion when this precipitation first begins? [PO4³] = + [References] Retry Entire Group 8 more group attempts remaining hp 4+ 16 g1 Quando a rede soci... fo fa NEW taarrow_forwardShop Trendy CHEM 1032Cengage S19 34 https://ng.cengage.com/static/nb/ui/evo/index.html?elSBN-9781305657571 &id-430934266&isnapshotld-10652508 s19 3.4 Cengage MInbox (112) DTAP Q Search this course L ions Use the References to access important values if needed for this question. The rate constant of the elementary reaction H2(g) +Br2(g) 2HBr(g) is k 4.45x10 L mol s at 250°C, and the reaction has an activation energy of 170 kJ mol (a) Compute the rate constant of the reaction at a temperature of 289°C L mol , 1 (b) After e (b) After equal concentrations of H, and Bry are mixed at 250o C, 6,03 10' s is required for half of them to be consumed. How long will itke to consume half of the reactants if an identical experiment is performed at 289°C? Submit Answer 3 question attempts remaining Autosaved at 9:31 AM Back 9:31 2/28arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY