
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Name the following compounds. I just need part CC and DD.
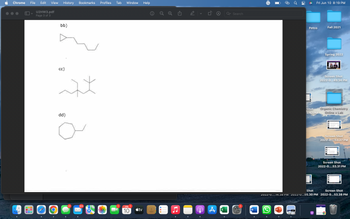
Transcribed Image Text:### Section: Organic Chemistry - Molecular Structures
#### Molecular Structures Overview
Below are three molecular structures, labeled as bb), cc), and dd), respectively. These diagrams illustrate various organic compounds.
1. **bb)**
- This structure represents a molecule with a cyclopropane ring at one end. Attached to the ring is a chain of carbon atoms with a series of single bonds. The molecular structure extends in a zigzag pattern, indicative of a typical alkane chain.
2. **cc)**
- This diagram illustrates a more complex organic molecule. It consists of a branched carbon chain. In the center, there is a tert-butyl group attached to the main chain. The structure highlights the complexity and branching that can occur in organic molecules.
3. **dd)**
- The final structure features a large ring, indicating a cyclooctane ring. Attached to this ring is an additional alkyl chain extending out from one of the carbon atoms in the ring. This structure showcases the cyclic nature of some organic compounds.
Each of these diagrams is a two-dimensional representation of a three-dimensional molecule.
Feel free to download the full lesson for an in-depth analysis of these structures and their chemical properties.
---
### Diagrams Explanations
These diagrams help students understand the different ways that carbon atoms can bond to form complex structures. The notation uses lines to represent bonds between carbon atoms, with vertices representing carbon atoms. This form of structural formula is commonly used in organic chemistry to simplify the representation of complex molecules.
For more details on molecular structure notation and how to interpret these diagrams, please refer to our advanced organic chemistry module.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Adding electrons to the skeleton by making single bonds between all bonded atoms gives Each hydrogen atom now has a pair of electrons, but each carbon has only 6 electrons. Adding a pair of electrons to each carbon gives the trial structure The number of electrons in the trial structure is ____. Since this number exceeds the number of available valence electrons, the structure is incorrect.arrow_forwardIn terms of organic chemistry how do you know if an atom has ionic bonds or covalent bonds and how do you know if it has just one of these or if it has both? Could you provide some examples of what an ionic bond looks like, covalent bond looks like, and what an atom would look like with both of these?arrow_forwarddrawing of the complete Lewis structure for vitamin b5, showing all atoms, bonds, lone pairs, and charges (as applicable). This structure must be hand-drawn by your group, not a printout from a webpage. c) Identification of all of the organic functional groups for your molecule, clearly labeled on your drawing of its Lewis structurearrow_forward
- What does a functional group do in an organic molecule?arrow_forwardExplain properly. Draw two different diagrams of the benzene molecule. Explain what each diagram shows, and state which diagram is thought to be more correct.arrow_forwardCan you help me to solve this Provide an explanation for why carbon-carbon bond lengths are different.arrow_forward
- Determine the functional group(s) for the following molecule (choose all that apply). a. aldehyde b. carboxylic acid c. acetal d. alcohol e. aromatic f. ester g. ketone h. hemiacetalarrow_forward1. Organic chemistry is most broadly defined as the chemistry of many compounds of carbon the chemistry of living systems the chemistry of the nonmetallic compounds the chemistry of substances produced by living systems. 2. An organic compound is best defined as carbon atoms covalently bonded to hydrogen atoms a compound containing carbon. a compound that is NOT produced in the laboratory. a compound produced an organism. 3. The simplest alkane is methane propane ethane monalkane Would like to know if my answers are correct. Bullet points in bold. thx :)arrow_forwardWhy does NH3 have a higher boiling point than BH3?To answer this question, draw out the Lewis structure for each molecule, and then draw out the VSEPR shape for each molecule. Also, Butane is more viscous (has higher intermolecular forces) than ethane. Why?arrow_forward
- What is organic chemistry? Identify how many covalent bonds each one of the following elements normally forms: Carbon: Nitrogen: Oxygen: Hydrogen: Halogens (F, Cl, Br, etc.):arrow_forwardDetermine the functional group(s) for the following molecule (choose all that apply). a. aldehyde b. ketone c. alcohol d. ester e. hemiacetal f. carboxylic acid g. acetal h. aromaticarrow_forward12. Identify (adraw a circle and label the class) the classes of organic molecules represented in the following molecule Ноarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning