
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:1. Molecule A below is a protected amino acid. Identify the correct amino acid.
Alanine
Proline
Tyrosine
Leucine
Glutamic acid
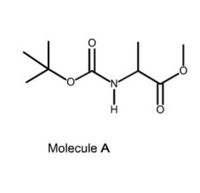
Transcribed Image Text:Molecule A
ネーエ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help with questions 2 and 3 pleasearrow_forward4: Draw structures from notations or notations from structures, for the following dipeptides. Look up in book for the structures of various amino acids and their names (and notations). (EOCQ 60) Hints for drawing a dipeptide from the specified amino acids. From the given symbols (Ser, Thr, etc), draw the structures for the amino acids in the specified order example, for Ser-Thr, draw Serine first and then Threonine). Then remove the OH from the first amin and one H (from the N atom) on the second amino acid. Then join the remaining fragments; that give the dipeptide formed. In these reactions, H2O is formed as a side product; but it is implied, and you have to show it in your answers. The following equation illustrates that. Here, R and R' are symbolic. In an actual question, you must the actual structures for the amino acids (Table 16.3 in the book). O H || | Н.N— CH— С-N-CH—С-ОН H,N-CH-C–OH + H,N-CH-Ċ–OH R' R. R' (H2O is a sid no need t Amino acid 2 dipeptide Amino acid 1 а. Cys-Ser…arrow_forwardWhat does the "alpha" indicate in "α-amino acid"? That the -COOH group is below the plane of the ring. That the amino group is the first group in the compound. That the alpha-carbon has a -NH2 attached. The the amino acid is the dominant form.arrow_forward
- 9. For the two amino acids below determine if each of them would be either on the inside or the outside of the tertiary structure of a protein. Explain your answer. CH3 H3C. A) B) HO. HO HO. NH2 NH2arrow_forward1. Which of the following statements is false? Many peptides come together to form polypeptides. They can form flat networks Proteins contain thousands of peptides cessibility: Good to go These can form an infinite number of configurations. O W (hparrow_forward3. An amino acid has a -CH,CH3 side chain. What type of side chain could form hydrogen bonds with this side chain? O a. -CH2-OH b. -CH,- C. -CH,-C-NH, d. none of the abovearrow_forward
- For the tripeptide ala-lys-phe a) Draw the structure of the peptide you would expect at pH 1. Show all lone pairs of electrons and show formal charges. Determine the charge on the tripeptide. b) Draw the structure of the peptide you would expect at pH 7. Show all lone pairs of electrons and show formal charges. Determine the charge on the tripeptide..arrow_forwardWhich amino acids could be referred to as derivatives of butanoic acid? Give their structures. Draw the amino acid in zwitterion form. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu.arrow_forwardConsidering the peptide structure on the image, label N-terminus and C-terminus, circle each of the peptide (amide) bonds, and provide the primary sequence of the protein using three letter abreviaciones for the amino acidsarrow_forward
- Questions 16 and 17 please.arrow_forwardLook at the amino acids shown below. Their side chains are highlighted, Which amino acids have polar side chains? *** H,N-Ç-COOH H,N- C-COOH COOH H,N-C-COOH CH2 CH, H. H-C-CH, NH3 ČH, H. H. OH Вarrow_forward09:56 7 < Question 18 of 19 The amino acid structure shown here is known as a(n) H₂N+ A) primary structure. B) secondary structure. C) C-terminus. D) N-terminus. Periodic Table Submitarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY