
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
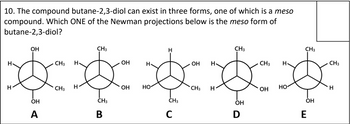
Transcribed Image Text:10. The compound butane-2,3-diol can exist in three forms, one of which is a meso
compound. Which ONE of the Newman projections below is the meso form of
butane-2,3-diol?
H
H
OH
OH
_ A
CH3
CH3
CH3
H
CH3 H
OH
H
OH H
CH3
H
CH3
CH3
H
OH
HO
CH3 H
но-
OH
H
CH3
CH3
OH
OH
B
C
D
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Following is a staggered conformation for one of the enantiomers of 2-bromobutane, and several purported Newman projections for this conformation. H H H3C Br H CH3 2-bromobutane H H (a) Is this (R)-2-bromobutane or (S)-2-bromobutane? CH3 CH3 Br H H H CH3 Br b CH3 H Br H CH3 CH3 H (b) Which of the following Newman projections is equivalent to the wedge-dash structure when viewed along the horizontal axis?arrow_forwardO O O H CH3 OH H H H H OH H H H 安安安安 H OH H HO H CH(CH3)2 CH(CH3)2 CH(CH3)2 CH(CH3)2 A C Which C2-C3 Newman projection represents (S)-4-methylpentan-2-ol? m A None of these OH .... (S)-4-methylpentan-2-ol B C Darrow_forwardDraw (15,25,3R)-1-bromo-3-chloro-2-fluorocyclohexane (shown below) in both its chair forms! Draw the Newman projection of the most stable conformation of (15,25,3R)-1-bromo- 3-chloro-2-fluorocyclohexane through carbons 1 and 2 and carbons 2 and 3. مه carrow_forward
- 11 11 HC–CHỊCH,CH2-CH A O=O KMnO4, H30 CH HO–C–CH,CH,CH,CH C ? O 11 HC-CH₂CH₂CH₂-C-OH B O HỌ-C–CH,CH,CH2-C-OH Darrow_forwardPART 1: For the decalin derivative below please identify the correct Newman projection: Please type your answer as a letter: A, B, C, or D. The answer for PART 1: Н H Н. A B CH3 CH3 Н CH3 CH3 Н အက်သလို လုံး Н. под Н OH CH3 OH H3C Н H3C H₂C H3C H3C Н Н The answer for PART 2: A CH3 OH E C CH3 CH3 OH "CH₂ CH3 H H3C. H3C Н H Н. H.C..... H3C OH OH CH3 PART 2: For the Newman projection below please identify the corresponding decalin derivative with the correct stereochemistry: Please type your answer as a letter: A, B, C, ..., or H. CH3 H B н Н OH F CH3 CH₂ OH он "CH3 OH CH₂ CH3 H3C H H₂C Н H3C Н H C D CH3 SASA SA CH3 H₂C CH3 H₂C" H3C" он CH3 H OH CH3 G CH3 CH3 CH3 ÕH D CH3 CH3 CH3 H H H3C_ Н.С Н CH3 CH₂ H OH H Н CH3arrow_forwardWhich of the following Newman projections represents (2R,3R)-dibromobutane?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning