
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
Transcribed Image Text:Bookmarks
Window
Help
ck
session.masteringchemistry.com
Molloy Portal
MyLab and Mastering
M MasteringChem
ion 11.5 while
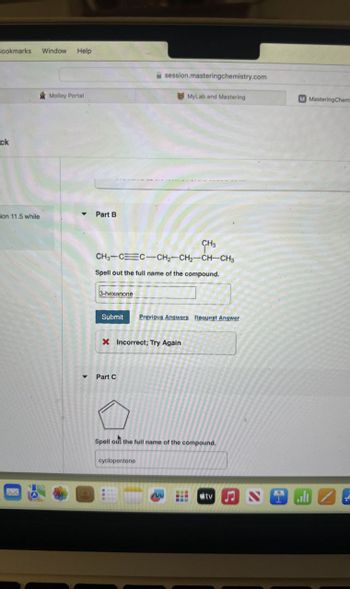
Part B
CH3
CH3-CC-CH2-CH2-CH-CH3
Spell out the full name of the compound.
3-hexanone
Submit Previous Answers Request Answer
X Incorrect; Try Again
Part C
Spell out the full name of the compound.
cyclopentene
M
tv
alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- j) k) combustion of pentane in oxygen (BE SURE TO BALANCE!) CH3 [H] [0] i CH₂CHCH₂CCH3 ? ? [0]. ?arrow_forward00:42 ← LLC0182 prob What isomers do the following pairs represent, explain your answer? A. B. CH₂CH₂ 其 Print Layout 1 H cis ||| Br H CH₂CH₂ Headings D Edit O CH₂CH₂ trans go ن e ...| 4% CH₂CH3 Br A) Share Read Aloud <arrow_forwardCompound C is? Compound C-DEPT 135 90 H3C I 70 CH3 CH 4 I 8 (ppm) CI CH₂CH₂CH-Cl CH₂ CH3 CH3-C-CH3 I Cl 1 I 30 10 CH3CH₂CH₂CH₂-Clarrow_forward
- Can u box ur final answer plz ??arrow_forwardHill C Chegg-Get OWLv2 | Ass Blackboard! Cengagenow.com/filrn/takeAssignment/takeCovalentActivity.do?locator assignment-take Mass= Water H₂O Ethanol CH3CH₂OH Chloroform CHC13 3:67 Benzene C6H6 2.53 Diethyl ether CH3 CH₂ OCH₂ CH3 2.02 Submit Answer Content g OWLv2 | CX [Review Topics] [References] Use the References to access important values if needed for this question. The freezing point of water is 0.00 °C at 1 atmosphere. How many grams of cobalt(II) bromide (218.7 g/mol), must be dissolved in 276.0 grams of water to reduce the freezing point by 0.450 °C? Refer to the table for the necessary boiling or freezing point constant. Solvent Formula Kb (°C/m) K(°C/m) 0.512 1.22 1.86 1.99 5.12 CengageNO ★ Retry Entire Group 4 more group attempts remaining New Tab D & ill Other Bookmarksarrow_forwardDraw the mechanism for the following reaction: Et - OH x+a CH3 CH₂ Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.arrow_forward
- Questions 1-3 refer to these structures: CH3CH2-O-CH2CH3 A CH3CH2CH2CH2CH₂CH₂OH B D C-H CH3CH2-O-C-CH2CH3 E || CH3-C-O-CH2CH2CH3 C O CH3-C-OH F CH3-C-CH2CH2CH3 Show cal Grations 1. a) List the letters for the structures that are esters. b) Circle the ester functional group in any esters. 2. a) Write the letters for the two compounds that could be used to make an ester by an esterification reaction. b) Write the reaction equation for this esterification reaction, showing the structures of all reactants and products. 3. Give the letters and the IUPAC names for the esters you identified in question 1.arrow_forwardIs this correct ??arrow_forwardsolve d,e,f in detailarrow_forward
- H3C CN 1) i-Bu₂AIH CH3 Ph 2) H3O+arrow_forwardpls helparrow_forward1-take [Review Topics] [References] Use the References to access important values if needed for this qu What is the IUPAC name of the following compound? CH3 CH3 M) CH3CHCHCHCH3 HO. (M) 2req 2req 2req M) Submit Answer Retry Entire Group 9 more group attempts remaining M) ts 2req ots 2req pts M)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
