
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
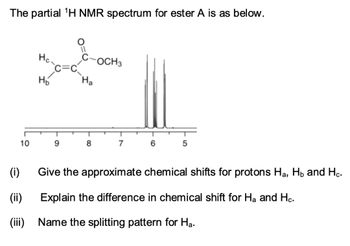
Transcribed Image Text:The partial ¹H NMR spectrum for ester A is as below.
10
(i)
(ii)
(iii)
Hc.
H₂
9
Ha
8
OCH 3
7
6
5
Give the approximate chemical shifts for protons H₂, H₂ and Hc.
Explain the difference in chemical shift for Ha and Hc.
Name the splitting pattern for Ha.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structure of the compound identified by the simulated 'H NMR and C NMR spectra. The molecular formula of the compound is C,0H,O. 12 (Blue numbers next to the lines in the 'H NMR spectra indicate the integration values.) H NMR 1H 2H 2H 2H 2H|| 3H 10 8. 4 (dd) g 13C NMR 220 200 180 160 140 120 100 80 60 40 20 8 (ppm) Deduce the structure from the spectra. Select Draw Rings More Erasearrow_forwardAnalyze the HNMR spectrum below and identify the compoundarrow_forward18. Proton NMR spectra are given together with molecular formula. Propose a structure that corresponds to each spectrum. (Hint: Find IHD first). Assign peaks to show which protons give rise to which peaks 10 10 10 (b) C4H8O₂ Offset: 2.4 ppm (b) C₂H100 9 (c) CsHgO₂ 9 8 8 7 7 6 6 6 5 8 (ppm) OHz 5 8 (ppm) 4 3.08 2.98 2.88 2.78 8 (ppm) 50Hz 4 4 3 2 1 0 0arrow_forward
- (d) Predict the multiplicity observed for H1 in the ¹H NMR spectrum of 4ax and 4eq. Ignore couplings to the OH proton and long-range couplings (more than 3 bonds). Assume the following 3- bond coupling constants between two axial protons Jax-ax, two equatorial protons Jea-eq, and one axial and one equatorial proton Jax-eq: Jax-ax = 10Hz Jeg eq = 4Hz Jax-eq = 3Hz 4ax: multiplicity of H1 = 4eq: multiplicity of H1 =_ (e) Use the coupling constants specified in part (d) and assume that 4ax and 4eq are formed in a 1:1 ratio. Sketch the multiplets that would be observed for H1 in both isomers according to scale: One multiplet in each box. Represent individual peaks in each multiplet with vertical lines. The height of each line should be proportional to the intensity of the peak in the multiplet. 2 Hz 2 HE #1 4eq 4ax H₂ OH OH CIarrow_forwardThe following NMR spectra were obtained from a compound with the molecular formula C4H6O. Use this information to predict its structure.arrow_forward10- If 3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which thevinylic proton at C2 is couples with both the C1 vinylic proton (J = 16 Hz) and the C3methylene protons (J = 8 Hz) Draw a tree diagram for the C2 proton signal.arrow_forward
- Annotate the NMR spectra of methyl 3-nitrobenzoatearrow_forwardAttached is the 1H NMR spec for phenylethylamine. Draw the structure of the amine on the spectrum, and identify each peak (e.g. a labeled H atom on your structure, TMS, etc.)arrow_forwardThe molecular formula of an unknown compound is C7H13BrO2, The strong peak at 1750 cm^-1 The proton NMR data is, 1.2d(6H, d), 4.2d(1H, t), 2.2d(2H, quintet), 4.93d(1H, septet),1.08d(3H,t).What is the name of the unknown compound? Isopropyl 2-bromobutanoate 1-bromopropyl isobutyrate ethyl 2-bromo-3-methylbutanoate 1-bromoethyl 3-methylbutanoatearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole