
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Absorbance at 453 nm
8393939
7 fl. oz./20 ml
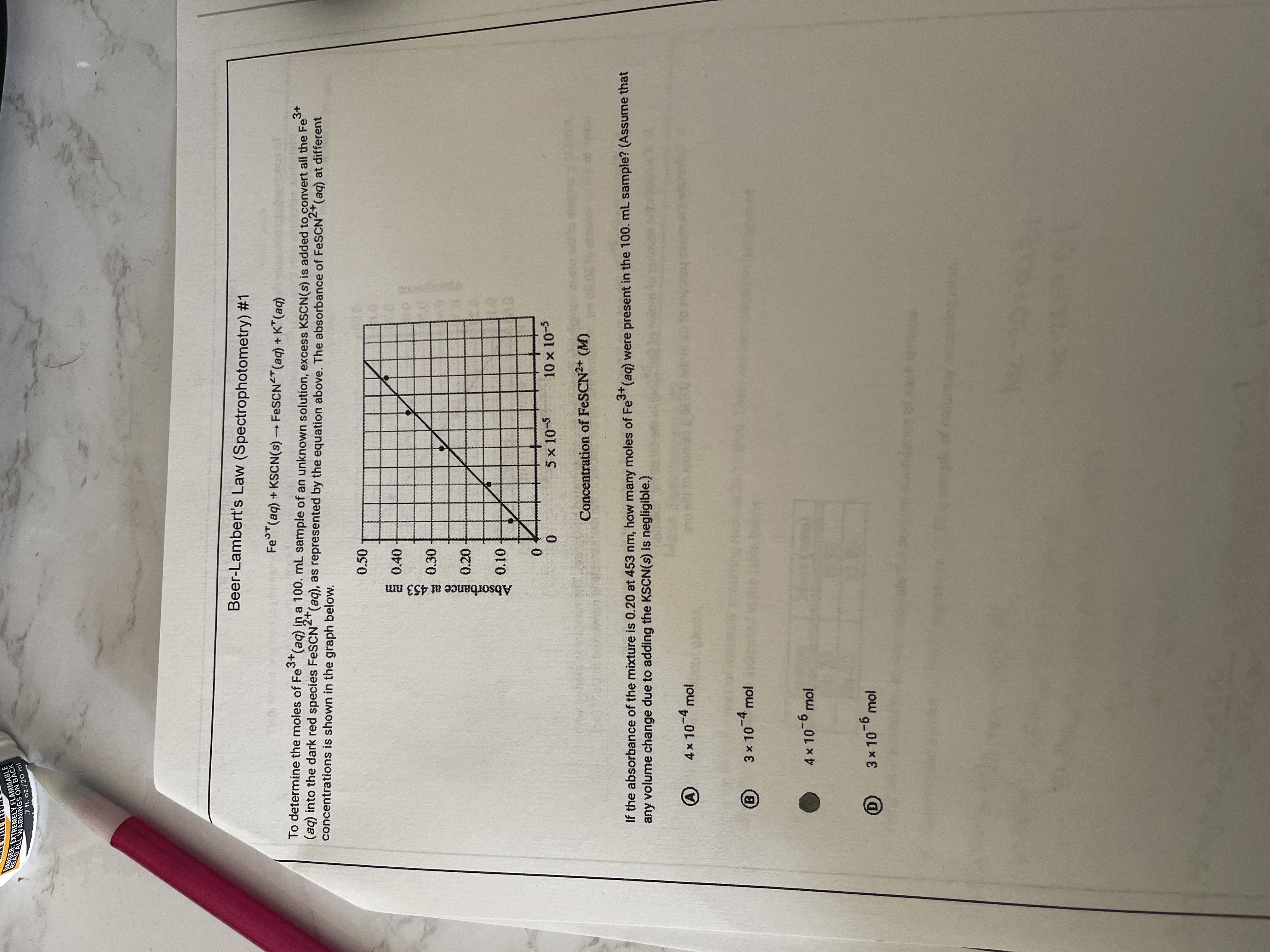
Beer-Lambert's Law (Spectrophotometry) #1
Fe (aq) + KSCN(s) FESCN "(aq) + K" (aq)
3+
To determine the moles of Fe (aq) in a 100. mL sample of an unknown solution, excess KSCN(s) is added to convert all the Fe
(aq) into the dark red species FeSCN"(aq), as represented by the equation above. The absorbance of FESCN"(aq) at different
concentrations is shown in the graph below.
2+
0.50
目0.40
0.30
0.20
01
0.
0.
5 x 10-5
10 x 10-5
Concentration of FESCN2+ (M)
If the absorbance of the mixture is 0.20 at 453 nm, haw many moles of Fe (aq) were present in the 100. mL sample? (Assume that
any volume change due to adding the KSCN(s) Iis negligible.)
3+,
4x10-4 mol
3 x 104 mol
(B)
4x 10-6 mol
3x 10-6 mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The concentration of a dilute aspirin solution is 0.000530 M 0.000530 M . Standard solutions of this compound were used to prepare a Beer's law plot which gives a slope of 1550.1 M −1 1550.1 M − 1 . What is the expected absorbance value for the aspirin solution?arrow_forwardSuppose that a solution has an absorbance of 0.250 at a wavelength of 450 nm. If the concentration of the solution is 22 μM, what is the value of the molar absorptivity? The data were taken with a standard 1-cm cuvette. molar absorptivity in (L cm^−1 mol^-1):arrow_forwardWhat would be a reasonable guess for the wavelength of maximum absorbance for FD&C Orange 2?arrow_forward
- For the aqueous [HgI4]complex Ky = 6.76 × 1029 at 25 °C. Suppose equal volumes of 0.0062 M Hg (NO3)2 solution and 0.42M Nal solution are mixed. Calculate the equilibrium molarity of aqueous Hg** ion. Round your answer to 2 significant digits. ☐ Marrow_forwardA series of standard iron-phenanthroline [Fe(phen)3]2+ complex ion solutions were prepared. The absorbance of each solution was measured at a wavelength of 510 mm. A calibration curve of absorbance vs. concentration was then plotted to obtain a linear equation of y = 0.0018x+0.0749. The absorbance of an unknown [Felphenly solution was measured to be 0.326. Calculate the concentration of the complex ion. NOTE! Report your answer as a WHOLE #. Type your answer...arrow_forwardYou want to determine the amount of iron(III) in acomplex salt. You make a 100.0 mL solution of the complex salt by dissolving 0.0670 g of complex salt with water to the mark on a 100.0 mL volumetric flask. You get an absorbance reading for the iron from the spectrometer of 0.334. The molar absorptivity is determined to be 17605 L/mole∙cm and the pathlength is 1.00 cm. Determine the molarity of iron(III) in solution. Determine the mass percentage of iron(III) in the complex salt.arrow_forward
- The absorbance of 10 mL of 0.0005 M Co2+ solution is 0.35. 10 mL of this solution mixed with 10 mL of the sample solution produces an absorption of 0.52. Calculate the concentration of Co2+ in the samplearrow_forwardAbsorbance measurements were taken on a series of Fe standards complexed with 1,10-phenanthroline. A plot of absorbance vs. concentration yielded a linear curve with the equation y=1.008e03x + 0.0395 (where y is the absorbance and x represents the Fe concentration in moles/L). Using this information calculate the Fe concentration (in ppm) in a 40.0-mL aliquot of ground water that produced an absorbance of 0.580.arrow_forwardYou did not allow enough time for the solution to reach equilibrium before measuring its absorbance. Explain how this will affect the reported value of Kc.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY