
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
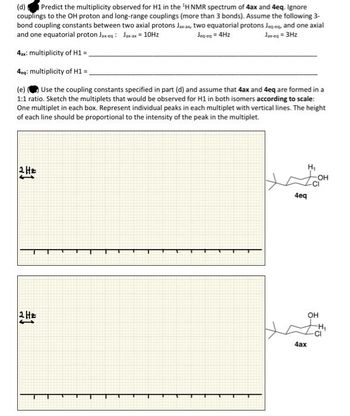
Transcribed Image Text:(d) Predict the multiplicity observed for H1 in the ¹H NMR spectrum of 4ax and 4eq. Ignore
couplings to the OH proton and long-range couplings (more than 3 bonds). Assume the following 3-
bond coupling constants between two axial protons Jax-ax, two equatorial protons Jea-eq, and one axial
and one equatorial proton Jax-eq: Jax-ax = 10Hz
Jeg eq = 4Hz
Jax-eq = 3Hz
4ax: multiplicity of H1 =
4eq: multiplicity of H1 =_
(e) Use the coupling constants specified in part (d) and assume that 4ax and 4eq are formed in a
1:1 ratio. Sketch the multiplets that would be observed for H1 in both isomers according to scale:
One multiplet in each box. Represent individual peaks in each multiplet with vertical lines. The height
of each line should be proportional to the intensity of the peak in the multiplet.
2 Hz
2 HE
#1
4eq
4ax
H₂
OH
OH
CI
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Draw the splitting pattern and assign and label the H of the spectrum of C4H8Oarrow_forwardDetermine the structure:A compound has an M+ peak of 104.Its proton NMR shows the following:1.3 ppm 3H triplet3.6 ppm 2H quartet4.1 ppm 2H singlet10.2 ppm 1H singlet, disappears when solvent is D2O. With the IR:arrow_forward¹H-NMR Spectra 3 M.F. = C₂H₁3NO 1 S 10 -00 8 6 PPM 4 N 21 3 ſ 2[2] -~ 2 3 jarrow_forward
- 17. Which of the compounds shown is consistent with the 13C NMR spectrum given? 80 OH I 70 60 HO. = || 50 40 PPM OH 30 20 IV OH 10 OHarrow_forwardIdentify the structure of the unknown compound from the given spectra. The letters on the NMR indicate the splitting pattern.@GMU 2020 Integrations: OA OB OD OE 7 5 -- DOM ►arrow_forwardFor the protons labeled Hª and Hb in the structure, predict the characteristics of their signals in the ¹H NMR spectrum: the approximate chemical shift, the splitting pattern, and the integration value. Ha a TOT a нь Нь Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY