
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
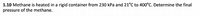
Transcribed Image Text:1.10 Methane is heated in a rigid container from 230 kPa and 21°C to 400°C. Determine the final
pressure of the methane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A nearly flat tire becomes noticeable warmer after it has been pumped up. Approximate this process as a reversible adiabatic compression of an ideal gas. Assume the initial pressure and temperature of the air before it is put in the tire to be pi = 1 atm and Ti = 25ºC and that CV,m = 3R/2. The final volume of the air in the tire is Vf = 2.00 L and the final pressure pf = 90 psi. Calculate the final temperature of the air in the tire (1 psi = 0.068046 atm, psi = pound per square inch). Also, calculate ∆U and ∆S during this process.arrow_forward1.12 Methane is heated in a rigid container from 230 kPa and 21oC to 4000C. Determine the final pressure of the methane.arrow_forward3. In an adiabatic cooling tower 500 kg/min hot water, is to be cooled from 50°C to 35°C by using air with 25°C dry bulb temperature and 15°C wet bulb temperature. The air leaves the tower with 32°C dry bulb temperature and 85% relative humidity. a. Show the points of inlet and outlet air on the PSYCHROMETRIC chart given below. Read the followings: Absolute humidity and Relative humidity of the inlet air. Absolute humidity and wet bulb temperature of the exit air. b. Calculate the volumetric flow rate of the air used. c. Why do we use cooling towers in process plant?arrow_forward
- Two hundred kJ of work is transferred to the air by means of a paddle wheel inserted into an insulated volume . If the initial pressure and temperature are 200 kPa and 100 °C, respectively, determine the final temperature and pressure.arrow_forwardCalculate the energy associated with a radiation having wavelength 3000 Å. Give the answer in kcal mol-¹ and also in kJ mol-¹.arrow_forwardWhy does the temperature remains the same at 0°C from1-3minutes ,event hough energy is being added the entire time?arrow_forward
- 4. The right figure shows a simple combustion calorimeter. The sample is ignited electrically. After a few minutes the temperature of the water and calorimeter is constant at AT higher than the starting temperature. Determine the heat of combustion of a sample from the following data: Sample mass Calorimeter mass Water mass 4 g 500 g 5000 g The heat of combustion is defined as = Sample Inlet 0 75 400 Temperature °F 70.0 Cvcalorimeter Cywater Ufinal products of combustion Pressure, psig Elevation, ft Velocity, ft/s AT Stirrer Uinitial fuel+oxygen Au combustion msample 5. A steady-flow water power plant has the following inlet and outlet conditions: Oxygen Outet 0 0 50 70.1 Water Thermometer Heavy steel bomb 0.12 cal/g °C 1.0 cal/g °C 5 °Carrow_forward2. A piston-cylinder device initially contains 0.8 kg of nitrogen gas at 160 kPa and 240 °C. The nitrogen gas is now expanded to a pressure of 100 kPa isothermally. Determine the work boundary during this process. 3. A frictionless piston-cylinder device contains 6 kg vapor at 600 kPa and 400 °C. When heat is transferred to the system, the vapor temperature is increasing up to 500 °C. Calculate the work net during this heat transfer process.arrow_forwardAir is contained in a vertical piston–cylinder assembly fitted with an electrical resistor. The atmosphere exerts a pressure of 101.325 kPa on the top of the piston, which has a mass of 50 kg and a face area of 0.10 m 2 . Electric current passes through the resistor, and the volume of the air slowly increases by 2 ft3 while its pressure remains constant. The mass of the air is 0.32 kg, and its specific internal energy increases by 20 Btu/lb. The air and piston are at rest initially and finally. The piston–cylinder material is a ceramic composite and thus a good insulator. Friction between the piston and cylinder wall can be ignored, and the local acceleration of gravity is g = 32.0 ft/s2 Determine the heat transfer from the resistor to the air, in Btu, for a system consisting of (a) the air alone, (b) the air and the piston. Also draw the schematic diagram. Hint: Refer to examplearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The