
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
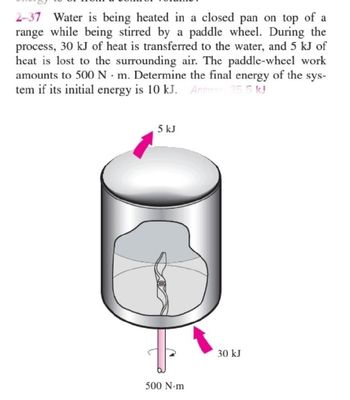
Transcribed Image Text:2-37 Water is being heated in a closed pan on top of a
range while being stirred by a paddle wheel. During the
process, 30 kJ of heat is transferred to the water, and 5 kJ of
heat is lost to the surrounding air. The paddle-wheel work
amounts to 500 Nm. Determine the final energy of the sys-
tem if its initial energy is 10 kJ. Answer 35.5 kJ
5 kJ
500 N-m
30 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Please make sure the answers are correct!arrow_forwardplease derivearrow_forward5. Ametal-clad heating element is of 8 mm diameter and of emissivity 0.95. The element is horizontally immersed in a water bath. The surface temperature of the metal is 260°C under steady state boiling conditions. Calculate the power dissipation per unit length for the heater if water is exposed to atmo- spheric pressure and is at uniform temperature. 1.75 kW/m]arrow_forward
- Consider how the Beer Heater impacts multiple aspects of distillation columns used in the bourbon industry. In these continuous distillation columns, name three three parameters that are coupled with each other?arrow_forwardA chiller cools liquid water for air-conditioning purposes. If 1.5 kg/s water at 20 °C, 100 kPa is cooled to 5 °C in a chiller. What should be the rate of heat transfer?arrow_forwardA shaft of 0.06-m diameter is heat treated in a gas-fired furnace whose gases is at 1200 K and provide a convection coefficient of 125 W/m² K. If the shaft enters the furnace at 300 K and stays in the furnace. (1) What is the centerline temperature (in K) of the shaft after 900 seconds? (2) What is the value of the dimensionless Fourier number? The shaft material has a density of 7000 kg/m², thermal conductivity of 50 W/m.K, and specific heat capacity 500 J/kg.K. Please show your work and assumptions.arrow_forward
- 3. Show the step-by-step process. Do not use shortcut methods. Make it as detailed as it can be. MAKE A DIAGRAM AND ENCODE THE ANSWER.arrow_forwardSprings in a suspension system of automobiles are made of steel rods heated and wound into coils while ductile. Consider steel rods (p = 7832 kg/m³, Cp = 434 J/kg K, k = 63.9 W/m-K) with a diameter of 2.5 cm and length of 1.27 m. The steel rods are heated in an oven with a uniform convection heat transfer coefficient of h = 20 W/m²-K. The steel rods are heated from an initial temperature of 20°C to the desired temperature of 450°C before being wound into coils. Determine the required ambient temperature in the oven if the steel rods must be heated to the desired temperature within 10 minutes. Prove the lump parameter analysis can be used.arrow_forwardA refrigeration system requires 1.5 kW of power for a refrigeration rate of 4 kW.(a) What is the coefficient of performance?(b) How much heat is rejected in the condenser?(c) If heat rejection is at 313.15 K (409C), what is the lowest temperature the system can possibly maintain?arrow_forward
- Answer: 24.187 °Carrow_forwardQuestion is from the class of Heat Transfer please show all steps, Thank you.arrow_forwardProblem 4. In order not to damage the resistance in the kettles, the difference between the temperature of the heater and the fluid should not exceed 25 °C. If the heat transfer area between the fluid and the heater is 78.5 cm² and the heat transfer coefficient is 400 W/m².K, find the maximum allowable power of the heater in order to boil water?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The