
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

Transcribed Image Text:1. The molar heat capacity of copper at 1 atm., in Joules/(K-mol) over the temperature
range 100 to 200 K can be approximated as Cm = 0.23 + 0.21 T - 0.00047 T² where T
is the temperature in Kelvin. Calculate AH and AS for heating 2.00 moles of copper from
100 K to 200 K at a constant pressure of 1 atm.
p.m
Expert Solution
arrow_forward
Step 1
Given data:
number of moles = 2.00 moles of copper
Cp,m =0.23+0.21T-0.00047T2
where, T is temperature
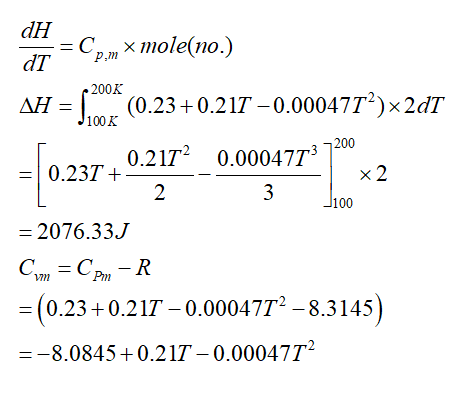
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The formula for dinitrogen monoxide (better known as nitrous oxide) is: (You will not be able to make subscripts and superscripts so just type in the letters and numbers. For example H20 for H₂0.1 [name]arrow_forwardCalculate the mass percent of hydrogen in C6H6 to 2 significant digits. [put only the 2 digit number, with a decimal pointarrow_forwardDo only one question if you cant do all of themarrow_forward
- Based on this. What mass of water was driven off by heating? (Grams)arrow_forwardIn order to synthesize the following ethers using the Willamson synthesis method, what would you need as starting material? DA. This ether cannot be synthesized using the Williamson method, but it can be done through electrohilic and nucleophilic substituions using fuming sulfuric acid as a catalyst. В. он Br OC. Br HO. D. and Brarrow_forward2 NazPO4 ( ) + _CaCl2 ( ) NacI ( )+ Ca3(PO4)2 ( ) The spectator ions in this equation (after balancing and filling in the states of matter) are (Be sure to include coefficients & chemical symbols, as well as the charges of the ions. No need to worry about making the charges subscript or typing ^ for these.) andarrow_forward
- M Apple Google Disney ESPN Yahoo! Biomedical Careers Program ☆ For the reaction Fe(s) +2HCl(aq)→→→→→FeCl₂(s) + H₂(g) AH° = -7.4 kJ and AS° = 107.9 J/K B Submit Answer Apple ☆ prod03-cnow-owl.cengagenow.com iCloud Yahoo Images Bing Google Wikipedia Facebook Twitter LinkedIn G b COWLv2... b D2L D2L D2L [Review Topics] [References] Use the References to access important values if needed for this question. D2L The maximum amount of work that could be done when 2.08 moles of Fe(s) react at 286 K, 1 atm is Assume that AH° and AS° are independent of temperature. Retry Entire Group 4 more group attempts remaining The Weather Channel C D2L Yelp TripAdvisor M kJ. +arrow_forwardHow many grams of oxygen (O) are present in a 6.41 g sample of potassium nitrate (KNO3)? Enter your answer in decimal form with the correct number of sig figs. Use the proper abbreviation for the units. NOTE: If you have taken chemistry before and know what diatomic means, do not take that into consideration. Use just O for this. There are reasons for this.arrow_forwardPlease help me answer thisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY