
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
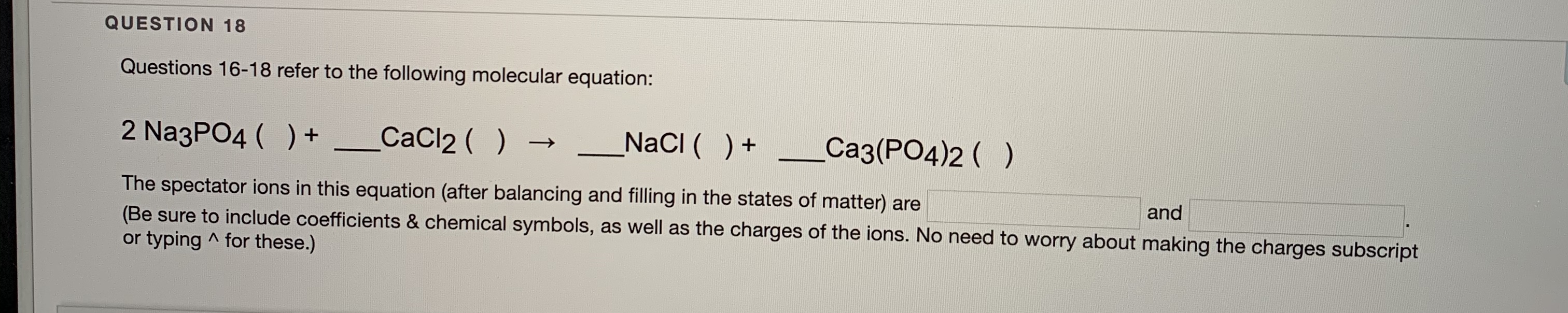
Transcribed Image Text:2 NazPO4 ( ) + _CaCl2 ( )
NacI ( )+
Ca3(PO4)2 ( )
The spectator ions in this equation (after balancing and filling in the states of matter) are
(Be sure to include coefficients & chemical symbols, as well as the charges of the ions. No need to worry about making the charges subscript
or typing ^ for these.)
and
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Iodine (I) and fluorine (F) form a series of binary compounds with the following compositions:Compound Mass % I Mass % F1 86.979 13.0212 69.007 30.9933 57.191 42.8094 48.829 51.171(a) Compute in each case the mass of fluorine that combines with 1.0000 g iodine.(b) By figuring out small whole-number ratios among thefour answers in part (a), show that these compoundssatisfy the law of multiple proportions.arrow_forwardCinnamic alcohol is used mainly in perfumery, particularly in soaps and cosmetics. Its molecular formula is C9H10O. (a) Calculate the percent composition by mass of C, H, and O in cinnamic alcohol. H % (b) How many molecules of cinnamic alcohol are contained in a 0.668-g sample? |× 10 molecules (Enter your answer in scientific notation)arrow_forwardConvert (8.808x10^24) molecules of water to grams of water. Avogadro's Number is (6.02x10^23). You must enter your answer in scientific notation. For example, 25 would be expressed as 2.5x10^1. Note: Your answer is assumed to be reduced to the highest power possible.arrow_forward
- Balance these chemical equations. (Use the lowest possible whole number coefficients.) (a) S8 + O2 → SO3 chemPad Help (b) NaNO3 → NaNO2 + O2 chemPad Help (c) C4H10 + O2 → CO2 + H2O chemPad Help (d) AgCl2 + H2 → Ag + HCl chemPad Help (e) Ga + H2SO4 → Ga2(SO4)3 + H2 chemPad Help (f) N2H4 + O2 → H2O2 + N2 chemPad Helparrow_forwardhttps://www.youtube.com/watch?v=2EQznGPZY5Aarrow_forwardA sample contains one or more of the ions: aluminum, calcium, copper(II), iron(III); upon addition of NH3, after centrifuging, it looks like the picture shown below. For each metal put in the correct letter: P = definitely present, A = definitely absent, U = uncertain, can't tell if present or absent. aluminum = , calcium = , copper = , iron = .arrow_forward
- Write balanced chemical eauations for the following reactions. The reaction of aqueous iron(III) nitrate and aqueous potassium carbonate to produce aqueous potassium nitrate and solid iron(II) carbonate.arrow_forwardUse the molar mass of Ca3(PO3)2 to calculate the number of formula units in 10.0 g of CP. Give your actual number. Use scientific notation with E representing the exponent and no spaces. For example, 1 mol = 6.022 x 1023 would be 6.022E23. (10.0 g CP)(––––––––––) = _________ mol CP (––––––––––) = _________ FUarrow_forwardConsider the following chemical reaction HCl (aq) + HgNO3 (aq) à Hg2Cl2 (s) + HNO3 (aq) If 20.0 grams of HCl is mixed with 30.0 grams of HgNO3 Determine the excess reactant The amount of the solid precipitate that was recovered equals to .................. grams , if the percentage yield from this reaction was determined to be 78.4%. Write the net ionic equation for the chemical reaction Determine the type of this chemical reactionarrow_forward
- The mass of the original mixture was 2.03g. The combined mass of the salt and the evaporating dish was 48.92g with the tare mass of the evaporating dish being 47.98g. The combined mass of the sand and watch glass and filter paper was 32.15g with the tare mass of the watch glass being 30.91g and of the filter paper being 0.28g. 1A) calculate the mass of the table salt recovered. 2A) Calculate the mass of sand recovered 3A)calculate the total mass of table salt and sand recovered. 4A)Calculate the percent table salt in recovered material. 5A) calculate the table salt in the original mixture.arrow_forwardA)Write the correct formula of copper (II) sulfate pentahydrate. (b) Calculate the theoretical percentage of water in Manganese (II) sulfate heptahydrate to four significant figures. You must show all calculations clearly to get full credit.arrow_forwardMass of 250 mL beaker (pe(Onju Mass of copper turnings + 250 mL beaker 101.73, O.47 68.32 69.7L Mass of copper turnings Teoeiq Hool nollasen svnb Mass of evaporating dish SnoitonoR Mass of evaporating dish + copper (before drying) Mass of evaporating dish + copper (after drying) EHOsn Mass of product (copper recovered) Seosig ool noitose1 s yitnsbi uov bib wok Percent Recover of Copper (Show Work): mass of product (recovered) mass of copper turnings eonsle (e)s(HO)O Percent Recovery = *100 %3Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY