
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:mail google.com
O O + O
A ALEKS-Yel Gutere-
C Chegg Search
M ino subject - ydgutier
Hulu Wateh
C Home Chegg.com
O This webpage is using significant memory. Closing it may improve the responsiveness of your Mac.
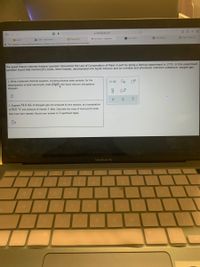
The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment
Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas.
1. Write a balanced chemical equation, including physical state symbols, for the
decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous
dioxygen.
O-0
?
2. Suppose 54.0 mL of dioxygen gas are produced by this reaction, at a temperature
of 50.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide
that must have reacted. Round your answer to 3 significant digits.
MacBook Air
G
K
I olo x
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Wine goes bad soon after opening because the ethanol CH3CH2OH in it reacts with oxygen gas O2 from the air to form water H2O and acetic acid CH3COOH , the main ingredient of vinegar. What mass of water is produced by the reaction of 2.32g of oxygen gas? Be sure your answer has FOUR significant digits.arrow_forwardLiquid hexane CH3CH24CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . What is the theoretical yield of water formed from the reaction of 79.3g of hexane and 213. g of oxygen gas? Be sure your answer has the correct number of significant digits in it.arrow_forward* Question Completion Status: A Moving to another question will save this response. Question 26 SHOW WORK Given the following reaction: CaC 2(s) + 2H 20 - C2H 2(g) + Ca(OH) 2(aq) Assume that you place 1.85 g of calcium carbide in excess water and collect the acetylene gas over water. Calculate the maximum amount of gas that should be produced in grams round answer to 3 decimal places omit trailing zeroes The actual volume of acetylene and water vapor collected is 810 ml at 30.5°C with a pressure of 557.524 mmHg. Calculate the actual amount of gas collected(in grams) round answer to 3 decimal places omit trailing zeroes The vapor pressure of water at 30.5°C is 32.35 mmHg. Calculate the percent yield of acetylene. round answer to 2 decimal places omit trailing zeros A Moving to another question will save this response.arrow_forward
- Liquid hexane CH3CH24CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . If 2.65g of water is produced from the reaction of 4.31g of hexane and 21.1g of oxygen gas, calculate the percent yield of water.Be sure your answer has the correct number of significant digits in it.arrow_forward(CONTINUED ON NEXT PAGE) 4. Let's say that the full volume of your flask is 325 ml. You heat the flask of air in a hot water bath at 89.0°C according to your thermometer. You then inverti into the cold water bath at 5.0°C Water enters the flask as the volume of the gas shrinks. a) Theoretically, what should the volume of the gas be after it sits in the cold water bath? b) You find that during your experiment that 65.0 mL of water has entered the flask. What is the experimental volume of the gas? c) What is your percent error for this measurement?arrow_forward4.35 L He/1 x 1 mole He/22.4 L He=0.19 moles He. Explain why the final answer of 0.19 moles makes more sense than 0.91 moles using the example answer attached to help.arrow_forward
- ce started, ne: 57 minutes, 35 seconds. Completion Status: A Moving to another question will save this response. Question 16 Magnesium reacts with hydrochloric acid to produce magnesium chloride and hydrogen gas: Mg(s) + 2 HCl(aq) MgCl2(aq) + H2(g) Suppose you need hydrogen to fill a balloon and you have 0.800 mol of magnesium and 2.00 moles of hydrochloric acid. How much hydrogen can you generate if the above reaction goes to completion? 6.00 moles 2.00 moles 0.400 moles 4.00 moles 0.800 moles 1.00 mole 6:38 PM 3/26/2020 P Type here to search hp end home ins prt sc delete (12 f9 144 f8 f7 f6 num f5 backspace lock & L. 8. {' pausearrow_forwardlculation questions require you to enter a numerical answer. You should not include units in your reported an ough you should note them in your calculations. Unless the problem specifically ask for an answer in the pro significant figures, it is often in your best interest to enter as many digits as your calculator will give you. 1 Question 1 Given the unbalanced chemical equation, C5H12(1) + O2(g) → CO(g) + H20(1), which one of the following correctly expresses the mole ratio between CO and oxygen? 5 mole CO/11 mole O2 O 1 mole CO/1 mole O2 O 10 mole CO/11 mole O2 O 10 mole CO/6.5 mole O2 Question 2arrow_forwardGaseous butane CH3CH22CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . Suppose 20. g of butane is mixed with 37.9 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forward
- Convert the following measurement. 3 g kg 1.6 x 10 x10 mol · L mol · dLarrow_forwardConvert the following measurement. -5 kg, g 7.6 x 10 x10 mol-dL mol·L Xarrow_forwardGaseous ethane CH3CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . Suppose 26.2 gof ethane is mixed with 35. g of oxygen. Calculate the minimum mass of ethane that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY